Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E1SW
|
|||
| Former ID |
DAP000600
|
|||
| Drug Name |
Acetazolamide
|
|||
| Synonyms |
Acetadiazol; Acetamidothiadiazolesulfonamide; Acetamox; Acetazolam; Acetazolamid; Acetazolamida; Acetazolamidum; Acetazolamine; Acetazoleamide; Acetozalamide; AkZol; ApoAcetazolamide; Atenezol; Cidamex; Dazamide; Defiltran; Dehydratin; Diacarb; Diakarb; Diamox; Didoc; Diluran; Diuramid; Diuramide; Diuriwas; Diutazol; Donmox; Duiramid; Edemox; Eumicton; Fonurit; Glauconox; Glaumox; Glaupax; Glupax; HumaZolamide; Natrionex; Nephramid; Nephramide; Phonurit; Storzolamide; Vetamox; Acetazolamide Apotex Brand; Acetazolamide Chiesi Brand; Acetazolamide Dioptic Brand; Acetazolamide Grin Brand; Acetazolamide ICN Brand; Acetazolamide Jumer Brand; Acetazolamide Llorens Brand; Acetazolamide Medphano Brand; Acetazolamide Novopharm Brand; Acetazolamide Orion Brand; Acetazolamide Wassermann Brand; Ak Zol; Apo Acetazolamide; Apotex Brand of Acetazolamide; Chiesi Brand of Acetazolamide; Ciba Vision Brand of Acetazolamide; DiamoxSequels; Dioptic Brand of Acetazolamide; Grin Brand of Acetazolamide; Huma Zolamide; ICN Brand of Acetazolamide; Jumer Brand of Acetazolamide; Llorens Brand of Acetazolamide; Medphano Brand of Acetazolamide; Monosodium Salt Acetazolamide; Novopharm Brand of Acetazolamide; Orion Brand of Acetazolamide; Storz Brand of Acetazolamide Preparation; Wassermann Brand of Acetazolamide; Wyeth Brand of Acetazolamide Preparation; A 6011; Carbonic anhydrase inhibitor 6063; Acetazolamida [INN-Spanish]; Acetazolamide (AAZ); Acetazolamide, Monosodium Salt; Acetazolamidum [INN-Latin]; Ak-Zol; Apo-Acetazolamide; Carbonic Anhydrase Inhibitor No. 6063; Diamox (TN); Diureticum-holzinger; Huma-Zolamide; SK-acetazolamide; Acetazolamide Sodium, (Sterile); Acetazolamide [INN:BAN:JAN]; Acetazolamide (JP15/USP/INN); 4-Diamox
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Glaucoma/ocular hypertension [ICD-11: 9C61; ICD-9: 365] | Approved | [1], [2] | |
| Therapeutic Class |
Anticonvulsants
|
|||
| Structure |
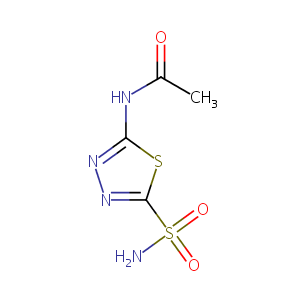 |
Download2D MOL |
||
| Formula |
C4H6N4O3S2
|
|||
| Canonical SMILES |
CC(=O)NC1=NN=C(S1)S(=O)(=O)N
|
|||
| InChI |
1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9)
|
|||
| InChIKey |
BZKPWHYZMXOIDC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 59-66-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9024, 428915, 619473, 855900, 5200439, 7847285, 7886028, 8149185, 8151366, 10321106, 10528898, 10589970, 11110756, 11110757, 11335192, 11360431, 11362772, 11365334, 11367896, 11371388, 11373697, 11376058, 11461403, 11466031, 11467151, 11483740, 11485662, 11487898, 11490100, 11491954, 11493832, 11533278, 12146089, 15121457, 17404603, 24278144, 26611589, 26679621, 26703043, 26713156, 26713159, 26713164, 26713489, 26715761, 26718123, 26747033, 26747034, 26751551, 29221174, 46393643
|
|||
| ChEBI ID |
CHEBI:27690
|
|||
| ADReCS Drug ID | BADD_D00025 ; BADD_D00026 | |||
| SuperDrug ATC ID |
S01EC01
|
|||
| SuperDrug CAS ID |
cas=000059665
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase I (CA-I) | Target Info | Modulator | [3] |
| KEGG Pathway | Nitrogen metabolism | |||
| Pathwhiz Pathway | Gastric Acid Production | |||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | |||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | |||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6792). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040195. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

