Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0C3CZ
|
|||
| Drug Name |
Aldoxorubicin
|
|||
| Synonyms |
1361644-26-9; ALDOXORUBICIN; Doxorubicin-EMCH; 151038-96-9; Aldoxorubicin (USAN); CHEMBL2107818; SCHEMBL15221892; OBMJQRLIQQTJLR-LBMCFUDOSA-N; ZINC163337436; AKOS030526452; CS-1186; HY-16261; D10383; W-5595; (8S)-1-Methoxy-6,8alpha,11-trihydroxy-8-[1-[2-[6-(2,5-dioxo-3-pyrroline-1-yl)hexanoyl]hydrazono]-2-hydroxyethyl]-10alpha-(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyloxy)-7,8,9,10-tetrahydronaphthacene-5,12-dione
Click to Show/Hide
|
|||
| Indication | Soft tissue sarcoma [ICD-11: 2B57; ICD-9: 171] | Phase 3 | [1], [2] | |
| Glioblastoma multiforme [ICD-11: 2A00.0; ICD-9: 191] | Phase 2 | [2] | ||
| Pancreatic ductal carcinoma [ICD-11: 2C10.0] | Phase 2 | [2] | ||
| Small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162.9] | Phase 2 | [1], [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2 | [3] | ||
| Company |
CytRx, Los Angeles, CA
|
|||
| Structure |
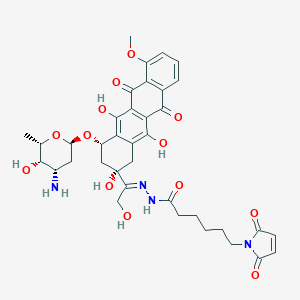 |
Download2D MOL |
||
| Formula |
C37H42N4O13
|
|||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=NNC(=O)CCCCCN6C(=O)C=CC6=O)CO)O)N)O
|
|||
| InChI |
1S/C37H42N4O13/c1-17-32(46)20(38)13-27(53-17)54-22-15-37(51,23(16-42)39-40-24(43)9-4-3-5-12-41-25(44)10-11-26(41)45)14-19-29(22)36(50)31-30(34(19)48)33(47)18-7-6-8-21(52-2)28(18)35(31)49/h6-8,10-11,17,20,22,27,32,42,46,48,50-51H,3-5,9,12-16,38H2,1-2H3,(H,40,43)/b39-23+/t17-,20-,22-,27-,32+,37-/m0/s1
|
|||
| InChIKey |
OBMJQRLIQQTJLR-USGQOSEYSA-N
|
|||
| CAS Number |
CAS 1361644-26-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Modulator | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT01580397) Pilot Phase 2 Study to Investigate the Preliminary Efficacy and Safety of INNO-206 in Advanced Pancreatic Cancer. U.S. National Institutes of Health. | |||
| REF 4 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

