Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0AL2K
|
|||
| Drug Name |
Irbesartan
|
|||
| Synonyms |
Aprovel; Avapro; Karvea; SR-47436; BMS-186295; BMS 186295; SR 47436; UNII-J0E2756Z7N; CHEMBL1513; YOSHYTLCDANDAN-UHFFFAOYSA-N; J0E2756Z7N; NCGC00095122-01; AK-57149; DSSTox_CID_3169; Irbesartan [USAN:INN]
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Investigative | [2], [3] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
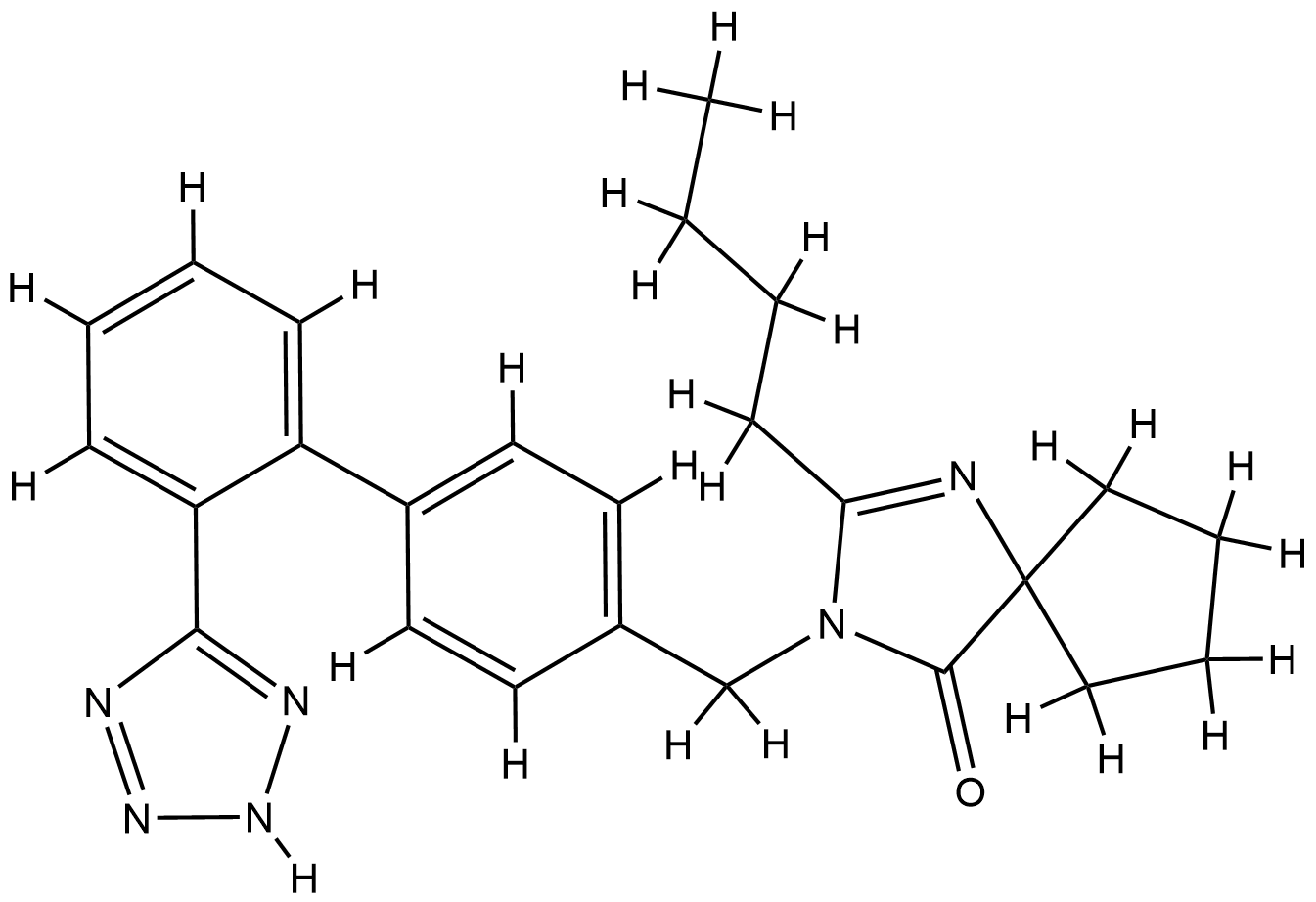 |
Download2D MOL |
||
| Formula |
C25H28N6O
|
|||
| Canonical SMILES |
CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5
|
|||
| InChI |
1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30)
|
|||
| InChIKey |
YOSHYTLCDANDAN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 138402-11-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:5959
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN type-1 angiotensin II receptor (AGTR1) | Target Info | Antagonist | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 203534. | |||
| REF 2 | Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001 Sep 20;345(12):851-60. Clinical Trial; | |||
| REF 3 | Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

