Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09JEJ
|
|||
| Former ID |
DNCL002399
|
|||
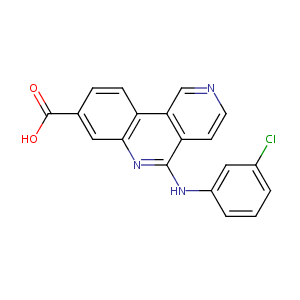
| Drug Name |
CX-4945
|
|||
| Synonyms |
1009820-21-6; Silmitasertib; CX-4945; 5-((3-Chlorophenyl)amino)benzo[c][2,6]naphthyridine-8-carboxylic acid; CX4945; CX-4945 (Silmitasertib); CX 4945; UNII-C6RWP0N0L2; 5-[(3-chlorophenyl)amino]-Benzo[c]-2,6-naphthyridine-8-carboxylic acid; 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid; C6RWP0N0L2; CHEMBL1230165; AK-82006; 5-[(3-Chlorophenyl)amino]benzo[c][2,6]naphthyridine-8-Carboxylic Acid; 5-((3-Chlorophenyl)amino)benzo-[c][2,6]naphthyridine-8-carboxylic acid; C19H12ClN3O2; W-204393
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus infection [ICD-11: 1D92; ICD-10: B34.2] | Phase 2 | [1] | |
| Cholangiocarcinoma [ICD-11: 2C12.10; ICD-10: C22.1] | Phase 1/2 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [3], [4] | ||
| Company |
Cylene Pharmaceuticals
|
|||
| Structure |
 |
Download2D MOL |
||
| Formula |
C19H12ClN3O2
|
|||
| Canonical SMILES |
C1=CC(=CC(=C1)Cl)NC2=NC3=C(C=CC(=C3)C(=O)O)C4=C2C=CN=C4
|
|||
| InChI |
1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25)
|
|||
| InChIKey |
MUOKSQABCJCOPU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1009820-21-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
49650657, 56164317, 103162249, 104246358, 123051134, 124360792, 125085740, 125164705, 125165310, 126661317, 126731254, 131549349, 135685370, 135685371, 135685390, 135723839, 136340300, 136349493, 136367624, 137230123, 137276036, 137525053, 143499362, 144115861, 152227610, 152234787, 152255064, 152258833, 152344058, 160647684, 160821183, 162011979, 162038030, 162202620, 163091488, 163372958, 164045277, 165241316, 172093670, 172913316, 174006751, 174561036, 180386992, 186020726, 189616233, 198951496, 203090868, 204389294, 223258866, 223375362
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Casein kinase II (CSNK2) | Target Info | Modulator | [5] |
| Casein kinase II alpha (CSNK2A1) | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Ribosome biogenesis in eukaryotes | |||
| NF-kappa B signaling pathway | ||||
| Wnt signaling pathway | ||||
| Adherens junction | ||||
| Tight junction | ||||
| Measles | ||||
| Herpes simplex infection | ||||
| Epstein-Barr virus infection | ||||
| NetPath Pathway | FSH Signaling Pathway | |||
| Pathway Interaction Database | BCR signaling pathway | |||
| Atypical NF-kappaB pathway | ||||
| DNA-PK pathway in nonhomologous end joining | ||||
| Presenilin action in Notch and Wnt signaling | ||||
| Role of Calcineurin-dependent NFAT signaling in lymphocytes | ||||
| E-cadherin signaling in the nascent adherens junction | ||||
| Lissencephaly gene (LIS1) in neuronal migration and development | ||||
| PDGFR-alpha signaling pathway | ||||
| Signaling mediated by p38-alpha and p38-beta | ||||
| Alpha-synuclein signaling | ||||
| Reactome | Condensation of Prometaphase Chromosomes | |||
| WikiPathways | Mitotic Prometaphase | |||
| BDNF signaling pathway | ||||
| TNF alpha Signaling Pathway | ||||
| L1CAM interactions | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04668209) Silmitasertib (CX-4945) in Patients With Severe Coronavirus Disease 2019 (COVID-19) (CX4945). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8126). | |||
| REF 4 | ClinicalTrials.gov (NCT01199718) Study of CX-4945 in Patients With Relapsed or Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||
| REF 5 | CX-4945, a selective inhibitor of casein kinase-2 (CK2), exhibits anti-tumor activity in hematologic malignancies including enhanced activity in chronic lymphocytic leukemia when combined with fludarabine and inhibitors of the B-cell receptor pathway. Leukemia. 2013 Oct;27(10):2094-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

