Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08EES
|
|||
| Former ID |
DIB006142
|
|||
| Drug Name |
XR-5000
|
|||
| Synonyms |
DACA; SN-22995
Click to Show/Hide
|
|||
| Indication | Colorectal cancer [ICD-11: 2B91.Z] | Phase 2 | [1] | |
| Company |
Xenova
|
|||
| Structure |
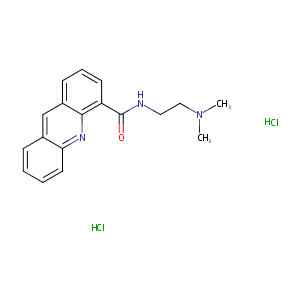 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase I (TOP1) | Target Info | Modulator | [1] |
| DNA topoisomerase II (TOP2) | Target Info | Modulator | [1] | |
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | |||
| WikiPathways | Integrated Pancreatic Cancer Pathway | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Phase II study of XR5000 (DACA) administered as a 120-h infusion in patients with recurrent glioblastoma multiforme. Ann Oncol. 2002 May;13(5):777-80. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

