Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08ABQ
|
|||
| Drug Name |
Acalabrutinib
|
|||
| Synonyms |
Acalabrutinib; 1420477-60-6; ACP-196; Calquence; UNII-I42748ELQW; I42748ELQW; Benzamide; Acalabrutinib [INN]; Acalabrutinib [USAN:INN]; Acalabrutinib (ACP-196); Calquence (TN); Benzamide, ACP-196; Acalabrutinib; Acalabrutinib(ACP196); GTPL8912; CHEMBL3707348; Acalabrutinib (JAN/USAN/INN); SCHEMBL14637368; EX-A881; ACP 196; WDENQI; Acalabrutinib
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | leukaemia [ICD-11: 2A60-2B33; ICD-9: 204] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 2 | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
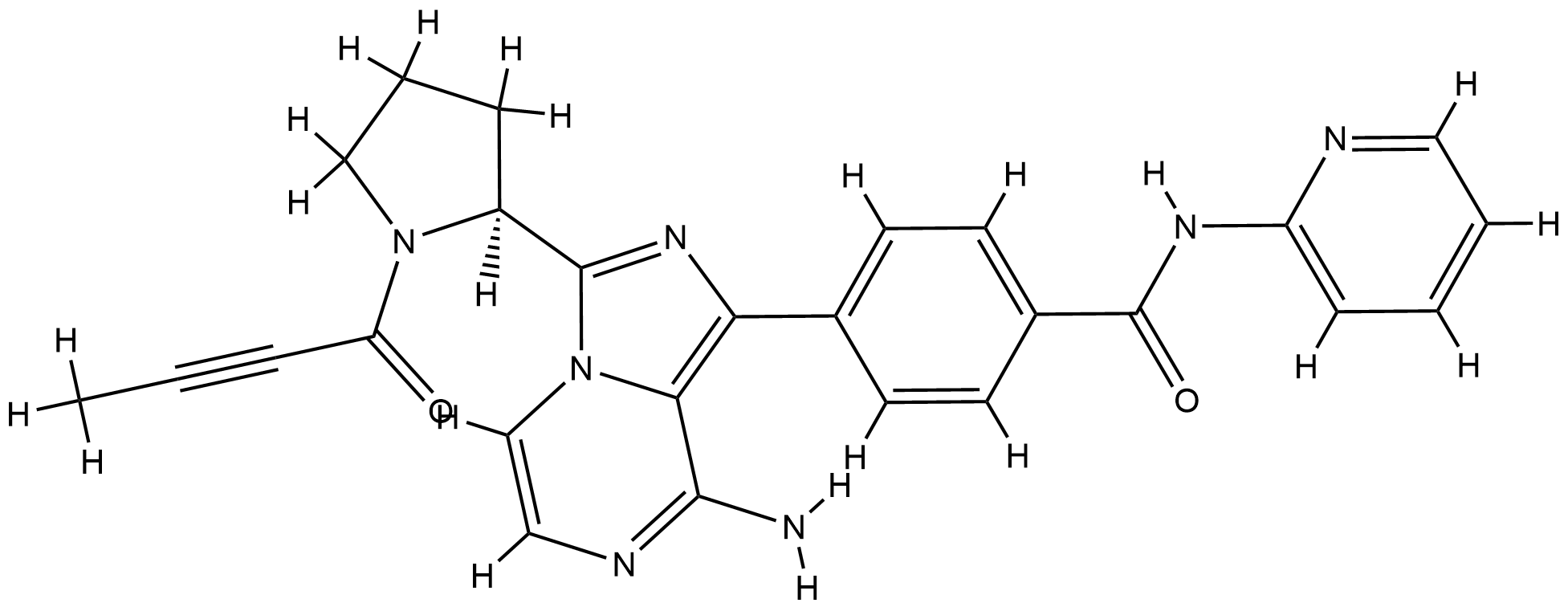 |
Download2D MOL |
||
| Formula |
C26H23N7O2
|
|||
| Canonical SMILES |
CC#CC(=O)N1CCCC1C2=NC(=C3N2C=CN=C3N)C4=CC=C(C=C4)C(=O)NC5=CC=CC=N5
|
|||
| InChI |
1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1
|
|||
| InChIKey |
WDENQIQQYWYTPO-IBGZPJMESA-N
|
|||
| CAS Number |
CAS 1420477-60-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN bruton tyrosine kinase (BTK) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 210259 | |||
| REF 2 | ClinicalTrials.gov (NCT04346199) Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19.. U.S. National Institutes of Health. | |||
| REF 3 | AstraZeneca initiates CALAVI clinical trial with Calquence against COVID-19 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

