Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07XVN
|
|||
| Former ID |
DAP000649
|
|||
| Drug Name |
Dexrazoxane
|
|||
| Synonyms |
24584-09-6; Zinecard; (S)-4,4'-(Propane-1,2-diyl)bis(piperazine-2,6-dione); Cardioxane; ICRF-187; Dexrazoxano; Dexrazoxanum; Dextrorazoxane; Dexrazoxanum [INN-Latin]; Dexrazoxano [INN-Spanish]; Desrazoxane; Eucardion; ADR 529; ICRF 187; (+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; Dexrazone; ADR-529; (+)-1,2-Bis(3,5-dioxo-1-piperazinyl)propane; HSDB 7319; UNII-048L81261F; NSC169780; dyzoxane; BRN 5759131; CHEBI:50223; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione; NSC 169780; AK-72797; Razoxanum; Cardioxane; Dyzoxane; Savene; TopoTect; Totect; Dexrazoxane HCl; Dexrazoxane hydrochloride; ICRF 187 hydrochloride; Cardioxane (TN); Dexrazoxane (TN); Totect (TN); Zinecard (TN); Dexrazoxane (USAN/INN); Dexrazoxane [USAN:BAN:INN]; Soluble ICRF (L-isosomer); Razoxane, (S)-Isomer; Totect, ICRF-187, Zinecard, Cardioxane, Dexrazoxane Hydrochloride;(+)-(S)-4,4'-Propylenedi-2,6-piperazinedione; (+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; (S)-(+)-1,2-Bis(3,5-dioxopiperazin-1-yl)propane; 2,6-Piperazinedione, 4,4'-(1-methyl-1,2-ethanediyl)bis-, (+)-(9CI); 4,4'-(2S)-propane-1,2-diyldipiperazine-2,6-dione; 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione hydrochloride; Icrf-187
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Breast cancer [ICD-11: 2C60-2C65] | Approved | [1], [2] | |
| Chemoprotection [ICD-11: N.A.] | Approved | [3], [4] | ||
| Respiratory tract disease [ICD-11: CB7Z; ICD-10: J00-J99; ICD-9: 460-519] | Investigative | [5] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Pfizer Pharmaceuticals
|
|||
| Structure |
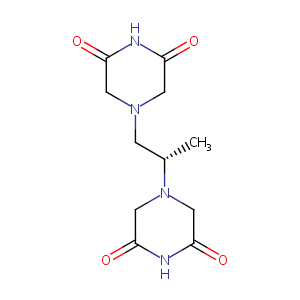 |
Download2D MOL |
||
| Formula |
C11H16N4O4
|
|||
| Canonical SMILES |
CC(CN1CC(=O)NC(=O)C1)N2CC(=O)NC(=O)C2
|
|||
| InChI |
1S/C11H16N4O4/c1-7(15-5-10(18)13-11(19)6-15)2-14-3-8(16)12-9(17)4-14/h7H,2-6H2,1H3,(H,12,16,17)(H,13,18,19)/t7-/m0/s1
|
|||
| InChIKey |
BMKDZUISNHGIBY-ZETCQYMHSA-N
|
|||
| CAS Number |
CAS 24584-09-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
442425, 831389, 8194593, 11405762, 14774896, 14872576, 17397819, 43127718, 46505982, 46506541, 50012896, 50139412, 57318172, 75683665, 93166226, 93619796, 103693192, 104350333, 117600767, 126670108, 127536650, 131307062, 134337939, 135029834, 136376430, 137002682, 137241718, 141868796, 144205809, 152035811, 160812421, 160871504, 160963727, 162174646, 163089322, 170464709, 172080045, 172092159, 172860861, 174007392, 174476563, 174560588, 175266611, 176484596, 176484943, 177748989, 178103902, 179117166, 184816244, 198945773
|
|||
| ChEBI ID |
CHEBI:50223
|
|||
| ADReCS Drug ID | BADD_D00633 ; BADD_D00634 | |||
| SuperDrug ATC ID |
V03AF02
|
|||
| SuperDrug CAS ID |
cas=024584096
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Modulator | [6] |
| DNA topoisomerase II beta (TOP2B) | Target Info | Inhibitor | [7] | |
| Panther Pathway | DNA replication | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7330). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076068. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000788) | |||
| REF 5 | Design and development of antisense drugs. Expert Opin. Drug Discov. 2008 3(10):1189-1207. | |||
| REF 6 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
| REF 7 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

