Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07QFP
|
|||
| Former ID |
DCL000314
|
|||
| Drug Name |
Teriflunomide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hyperlipidaemia [ICD-11: 5C80; ICD-10: E78.5] | Approved | [1] | |
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Approved | [2], [3] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 3 | [2], [4] | ||
| Hepatitis B virus infection [ICD-11: 1E51.0; ICD-10: B18.1] | Phase 1 | [1] | ||
| Company |
Sanofi-Aventis
|
|||
| Structure |
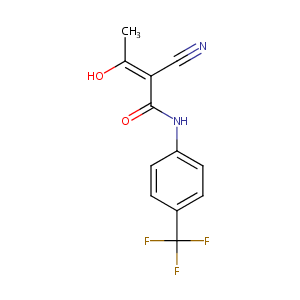 |
Download2D MOL |
||
| Formula |
C12H9F3N2O2
|
|||
| Canonical SMILES |
CC(=C(C#N)C(=O)NC1=CC=C(C=C1)C(F)(F)F)O
|
|||
| InChI |
1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,18H,1H3,(H,17,19)/b10-7-
|
|||
| InChIKey |
UTNUDOFZCWSZMS-YFHOEESVSA-N
|
|||
| CAS Number |
CAS 163451-81-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
605680, 836841, 8020778, 12014666, 15221873, 17182873, 29223012, 32961983, 36887811, 39470329, 49896832, 53790606, 57363933, 77206778, 77206779, 77206780, 93311044, 99311205, 103252419, 103929187, 104304838, 104633246, 113986292, 131294182, 134223898, 134338701, 134338970, 134340294, 134340452, 135074184, 135263629, 135626890, 137116564, 143267135, 152134192, 160645856, 162254943, 163414519, 163620868, 163686197, 164178177, 164835947, 172919649, 175266318, 176251501, 178103450, 180100588, 180371831, 187072836, 198993049
|
|||
| ChEBI ID |
CHEBI:68540
|
|||
| SuperDrug ATC ID |
L04AA31
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Emerging drugs in peripheral arterial disease. Expert Opin Emerg Drugs. 2006 Mar;11(1):75-90. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6844). | |||
| REF 3 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | |||
| REF 4 | Multiple sclerosis: current and future treatment options. Endocr Metab Immune Disord Drug Targets. 2007 Dec;7(4):292-9. | |||
| REF 5 | Expression and characterization of E. coli-produced soluble, functional human dihydroorotate dehydrogenase: a potential target for immunosuppression. J Mol Microbiol Biotechnol. 1999 Aug;1(1):183-8. | |||
| REF 6 | Species-related inhibition of human and rat dihydroorotate dehydrogenase by immunosuppressive isoxazol and cinchoninic acid derivatives. Biochem Pharmacol. 1998 Nov 1;56(9):1259-64. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

