Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07LUJ
|
|||
| Former ID |
DNC007423
|
|||
| Drug Name |
URSOLIC ACID
|
|||
| Synonyms |
Ursolic acid; Prunol; Malol; Urson; 77-52-1; 3beta-Hydroxyurs-12-en-28-oic acid; (3beta)-3-Hydroxyurs-12-en-28-oic acid; 3beta-Hydroxy-12-ursen-28-ic acid; UNII-P3M2575F3F; CHEBI:9908; CCRIS 7123; EINECS 201-034-0; CHEMBL169; Merotaine; AI3-03109; NSC4060; P3M2575F3F; NSC-4060; Urs-12-en-28-oic acid, 3-hydroxy-, (3beta)-; HNMR; .beta.-Ursolic acid; NSC 4060; NSC 167406
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Metabolic syndrome x [ICD-11: 5C50-5D2Z] | Phase 2 | [1] | |
| Structure |
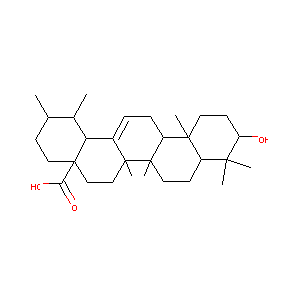 |
Download2D MOL |
||
| Formula |
C30H48O3
|
|||
| Canonical SMILES |
CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1C)C)C(=O)O
|
|||
| InChI |
1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1
|
|||
| InChIKey |
WCGUUGGRBIKTOS-GPOJBZKASA-N
|
|||
| CAS Number |
CAS 77-52-1
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:9908
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02337933) Effect of Ursolic Acid Administration on Insulin Sensitivity and Metabolic Syndrome. U.S. National Institutes of Health. | |||
| REF 2 | 11beta-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational a... Bioorg Med Chem. 2010 Feb 15;18(4):1507-15. | |||
| REF 3 | Synthesis of 3-deoxypentacyclic triterpene derivatives as inhibitors of glycogen phosphorylase. J Nat Prod. 2009 Aug;72(8):1414-8. | |||
| REF 4 | DNA polymerase beta inhibitors from Baeckea gunniana. J Nat Prod. 1999 Dec;62(12):1624-6. | |||
| REF 5 | Synthesis of benzoyl phenyl benzoates as effective inhibitors for phospholipase A2 and hyaluronidase enzymes. Bioorg Med Chem Lett. 2005 Sep 15;15(18):4100-4. | |||
| REF 6 | Cytotoxic and PTP1B inhibitory activities from Erythrina abyssinica. Bioorg Med Chem Lett. 2009 Dec 1;19(23):6745-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

