Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07DAU
|
|||
| Former ID |
DIB004342
|
|||
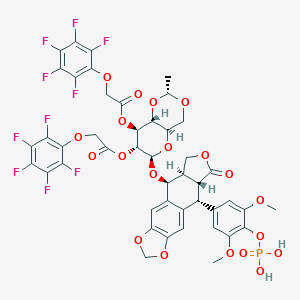
| Drug Name |
Tafluposide
|
|||
| Synonyms |
F-11782
Click to Show/Hide
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [1] | |
| Company |
Pierre Fabre SA
|
|||
| Structure |
 |
Download2D MOL
|
||
| Formula |
C45H35F10O20P
|
|||
| Canonical SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)OP(=O)(O)O)OC)OC(=O)COC8=C(C(=C(C(=C8F)F)F)F)F)OC(=O)COC9=C(C(=C(C(=C9F)F)F)F)F
|
|||
| InChI |
1S/C45H35F10O20P/c1-13-64-9-22-39(70-13)42(72-23(56)10-65-40-33(52)29(48)27(46)30(49)34(40)53)43(73-24(57)11-66-41-35(54)31(50)28(47)32(51)36(41)55)45(71-22)74-37-16-7-19-18(68-12-69-19)6-15(16)25(26-17(37)8-67-44(26)58)14-4-20(62-2)38(21(5-14)63-3)75-76(59,60)61/h4-7,13,17,22,25-26,37,39,42-43,45H,8-12H2,1-3H3,(H2,59,60,61)/t13-,17+,22-,25-,26+,37-,39-,42+,43-,45+/m1/s1
|
|||
| InChIKey |
RTJVUHUGTUDWRK-CSLCKUBZSA-N
|
|||
| CAS Number |
CAS 179067-42-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase I (TOP1) | Target Info | Modulator | [2] |
| DNA topoisomerase II (TOP2) | Target Info | Modulator | [2] | |
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | |||
| WikiPathways | Integrated Pancreatic Cancer Pathway | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012192) | |||
| REF 2 | Ex vivo effects of the dual topoisomerase inhibitor tafluposide (F 11782) on cells isolated from fresh tumor samples taken from patients with cancer. Anticancer Drugs. 2003 Jul;14(6):467-73. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

