Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07BXE
|
|||
| Former ID |
DIB005578
|
|||
| Drug Name |
Daniquidone
|
|||
| Synonyms |
Batracylin
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Lymphoma [ICD-11: 2A80-2A86; ICD-9: 202.8, 208.9] | Phase 1 | [1] | |
| Company |
National Cancer Institute
|
|||
| Structure |
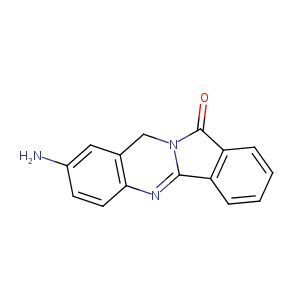 |
Download2D MOL |
||
| Formula |
C15H11N3O
|
|||
| Canonical SMILES |
C1C2=C(C=CC(=C2)N)N=C3N1C(=O)C4=CC=CC=C43
|
|||
| InChI |
1S/C15H11N3O/c16-10-5-6-13-9(7-10)8-18-14(17-13)11-3-1-2-4-12(11)15(18)19/h1-7H,8,16H2
|
|||
| InChIKey |
SRIOCKJKFXAKHK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 67199-66-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase I (TOP1) | Target Info | Modulator | [2] |
| DNA topoisomerase II (TOP2) | Target Info | Modulator | [2] | |
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Caspase Cascade in Apoptosis | |||
| WikiPathways | Integrated Pancreatic Cancer Pathway | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00450502) Safety of Batracylin in Patients With Solid Tumors and Lymphomas. U.S. National Institutes of Health. | |||
| REF 2 | Pharmacogenetically driven patient selection for a first-in-human phase I trial of batracylin in patients with advanced solid tumors and lymphomas.Cancer Chemother Pharmacol.2013 Oct;72(4):917-23. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

