Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06OMW
|
|||
| Former ID |
DAP001107
|
|||
| Drug Name |
Rasagiline
|
|||
| Synonyms |
RAS; Rasagiline [INN]; Azilect (TN); Rasagiline (INN); (1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-amine; (1R)-N-prop-2-ynyl-2,3-dihydro-1H-inden-1-amine; (R)-N-2-Propynyl-1-indanamine; 1H-Inden-1-amine, 2,3-dihydro-N-2-propynyl-, (1R)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-9: 332] | Approved | [1], [2] | |
| Skin cancer [ICD-11: 2C30-2C37] | Patented | [3] | ||
| Skin imperfections [ICD-11: EK71; ICD-10: L91.8] | Patented | [3] | ||
| Therapeutic Class |
Antiparkinson Agents
|
|||
| Company |
Teva Pharmaceutical Industries Ltd
|
|||
| Structure |
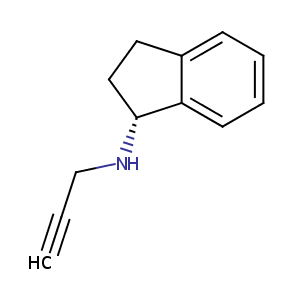 |
Download2D MOL |
||
| Formula |
C12H13N
|
|||
| Canonical SMILES |
C#CCNC1CCC2=CC=CC=C12
|
|||
| InChI |
1S/C12H13N/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12/h1,3-6,12-13H,7-9H2/t12-/m1/s1
|
|||
| InChIKey |
RUOKEQAAGRXIBM-GFCCVEGCSA-N
|
|||
| CAS Number |
CAS 136236-51-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7890222, 7980093, 10056118, 15120458, 15219503, 36118694, 46506045, 46511554, 50070333, 50071312, 56313239, 61269083, 80749168, 92713124, 96025155, 103233337, 111619048, 121277906, 121362276, 126573831, 126625000, 126656198, 126667056, 126732599, 129184029, 132554079, 134338352, 134358863, 135228398, 135611108, 135683150, 136340364, 136373590, 137001346, 142523977, 143493292, 144076117, 151977094, 152165036, 152234171, 160816897, 160964654, 162011569, 162204826, 163090554, 164848146, 172090451, 172859758, 174479357, 175267772
|
|||
| ChEBI ID |
CHEBI:63620
|
|||
| ADReCS Drug ID | BADD_D01916 ; BADD_D02441 | |||
| SuperDrug ATC ID |
N04BD02
|
|||
| SuperDrug CAS ID |
cas=136236516
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Monoamine oxidase type B (MAO-B) | Target Info | Inhibitor | [4] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation via tryptamine | ||||
| Dopamine degradation | ||||
| Putrescine degradation III | ||||
| Noradrenaline and adrenaline degradation | ||||
| KEGG Pathway | Glycine, serine and threonine metabolism | |||
| Arginine and proline metabolism | ||||
| Histidine metabolism | ||||
| Tyrosine metabolism | ||||
| Phenylalanine metabolism | ||||
| Tryptophan metabolism | ||||
| Drug metabolism - cytochrome P450 | ||||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Dopaminergic synapse | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| 5-Hydroxytryptamine degredation | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| WikiPathways | Tryptophan metabolism | |||
| Dopamine metabolism | ||||
| Phase 1 - Functionalization of compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6641). | |||
| REF 2 | Novel pharmacological targets for the treatment of Parkinson's disease. Nat Rev Drug Discov. 2006 Oct;5(10):845-54. | |||
| REF 3 | Novel monoamine oxidase inhibitors: a patent review (2012 - 2014).Expert Opin Ther Pat. 2015 Jan;25(1):91-110. | |||
| REF 4 | Glyceraldehyde-3-phosphate dehydrogenase-monoamine oxidase B-mediated cell death-induced by ethanol is prevented by rasagiline and 1-R-aminoindan. Neurotox Res. 2009 Aug;16(2):148-59. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

