Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06KSV
|
|||
| Former ID |
DIB000328
|
|||
| Drug Name |
Omigapil
|
|||
| Synonyms |
Omigapil); CGP-3466; CGP-3499B; Neuroprotectant, Novartis; Neuroprotectant, Santhera; Nootropic agent, Novartis; Nootropicagent, Santhera; SNT-317; TCH-346; TCH-346B
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Lateral sclerosis [ICD-11: 8B61] | Phase 2 | [1] | |
| Muscular dystrophy [ICD-11: 8C70; ICD-10: G71.0] | Phase 1 | [2], [3] | ||
| Company |
Novartis AG
|
|||
| Structure |
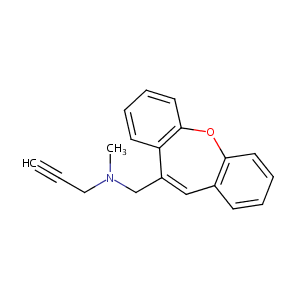 |
Download2D MOL |
||
| Formula |
C19H17NO
|
|||
| Canonical SMILES |
CN(CC#C)CC1=CC2=CC=CC=C2OC3=CC=CC=C31
|
|||
| InChI |
1S/C19H17NO/c1-3-12-20(2)14-16-13-15-8-4-6-10-18(15)21-19-11-7-5-9-17(16)19/h1,4-11,13H,12,14H2,2H3
|
|||
| InChIKey |
QLMMOGWZCFQAPU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 181296-84-4
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:41778
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Target Info | Modulator | [4] |
| BioCyc | Superpathway of conversion of glucose to acetyl CoA and entry into the TCA cycle | |||
| Gluconeogenesis | ||||
| NADH repair | ||||
| Glycolysis | ||||
| KEGG Pathway | Glycolysis / Gluconeogenesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| Carbon metabolism | ||||
| Biosynthesis of amino acids | ||||
| HIF-1 signaling pathway | ||||
| Alzheimer's disease | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| IL2 Signaling Pathway | ||||
| TSH Signaling Pathway | ||||
| Panther Pathway | Glycolysis | |||
| Huntington disease | ||||
| Pathwhiz Pathway | Glycerol Phosphate Shuttle | |||
| Glycolysis | ||||
| Gluconeogenesis | ||||
| Mitochondrial Electron Transport Chain | ||||
| Warburg Effect | ||||
| Pathway Interaction Database | Validated targets of C-MYC transcriptional activation | |||
| Reactome | Glycolysis | |||
| Gluconeogenesis | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Glycolysis and Gluconeogenesis | ||||
| Alzheimers Disease | ||||
| Cori Cycle | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00649961) Melatonin As A Novel Neuroprotectant In Preterm Infants- Dosage Study. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3887-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

