Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05WQF
|
|||
| Former ID |
DNC011169
|
|||
| Drug Name |
2-Hydroxycinnamic acid
|
|||
| Synonyms |
2-Hydroxy Cinnamic Acid; 3-(2-hydroxyphenyl)prop-2-enoic acid; Orthocumarsaure; ACMC-1BBQC; AC1L1YJR; 2-HYDROXYCINNAMICACID; CTK1F7842; CTK1H2007; CTK1B5135; PMOWTIHVNWZYFI-UHFFFAOYSA-N; MolPort-006-109-312; HMS3604C08; KS-00000X9C; EINECS 209-500-5; AKOS026677434; MCULE-7485332494; SY048367; FT-0612592; FT-0612591; MFCD00004379 (97%); 2-Propenoic acid, 3-(hydroxyphenyl)-, (Z)-; 2-Propenoic acid, 3-(hydroxyphenyl)-, (2E)-; 38094-41-6
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
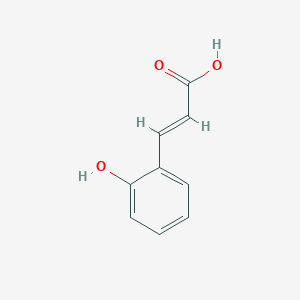 |
Download2D MOL |
||
| Formula |
C9H8O3
|
|||
| Canonical SMILES |
C1=CC=C(C(=C1)C=CC(=O)O)O
|
|||
| InChI |
1S/C9H8O3/c10-8-4-2-1-3-7(8)5-6-9(11)12/h1-6,10H,(H,11,12)/b6-5+
|
|||
| InChIKey |
PMOWTIHVNWZYFI-AATRIKPKSA-N
|
|||
| CAS Number |
CAS 614-60-8
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:18125
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase I (CA-I) | Target Info | Inhibitor | [1] |
| Carbonic anhydrase II (CA-II) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Nitrogen metabolism | |||
| Proximal tubule bicarbonate reclamation | ||||
| Collecting duct acid secretion | ||||
| Gastric acid secretion | ||||
| Pancreatic secretion | ||||
| Bile secretion | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| EGFR1 Signaling Pathway | ||||
| Pathwhiz Pathway | Gastric Acid Production | |||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | |||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | |||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett. 2010 Sep 1;20(17):5050-3. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

