Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04QJY
|
|||
| Former ID |
DNC012015
|
|||
| Drug Name |
KELATORPHAN
|
|||
| Synonyms |
kelatorphan; 92175-57-0; UNII-46BBW2U5D6; n-[(2r)-2-benzyl-4-(hydroxyamino)-4-oxobutanoyl]-l-alanine; 46BBW2U5D6; (3-(N-Hydroxy)carboxamido-2-benzylpropanoyl)alanine; L-Alanine, N-(4-(hydroxyamino)-1,4-dioxo-2-(phenylmethyl)butyl)-, (R)-; 3b7u; AC1L3XO7; CHEMBL85320; SCHEMBL7384459; BDBM92500; DTXSID90238911; ZINC6020153; DB08040; HY-10827; KB-78017; LS-186814; LS-187482; CS-0002846; (2S)-2-[[(2R)-2-benzyl-4-(hydroxyamino)-4-oxobutanoyl]amino]propanoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
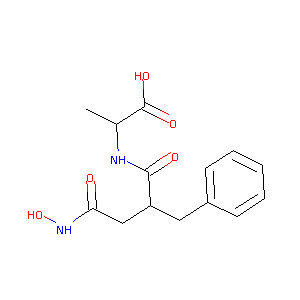 |
Download2D MOL |
||
| Formula |
C14H18N2O5
|
|||
| Canonical SMILES |
CC(C(=O)O)NC(=O)C(CC1=CC=CC=C1)CC(=O)NO
|
|||
| InChI |
1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1
|
|||
| InChIKey |
OJCFZTVYDSKXNM-GXSJLCMTSA-N
|
|||
| CAS Number |
CAS 92175-57-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aminopeptidase N (ANPEP) | Target Info | Inhibitor | [1] |
| Glutamyl aminopeptidase (ENPEP) | Target Info | Inhibitor | [1] | |
| BioCyc | Glutathione-mediated detoxification | |||
| KEGG Pathway | Glutathione metabolism | |||
| Metabolic pathways | ||||
| Renin-angiotensin system | ||||
| Hematopoietic cell lineage | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Pathwhiz Pathway | Glutathione Metabolism | |||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| Reactome | Metabolism of Angiotensinogen to Angiotensins | |||
| WikiPathways | Metabolism of Angiotensinogen to Angiotensins | |||
| Cardiac Progenitor Differentiation | ||||
| miR-targeted genes in squamous cell - TarBase | ||||
| miR-targeted genes in muscle cell - TarBase | ||||
| miR-targeted genes in lymphocytes - TarBase | ||||
| miR-targeted genes in leukocytes - TarBase | ||||
| Glutathione metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Retro-inverso concept applied to the complete inhibitors of enkephalin-degrading enzymes. J Med Chem. 1988 Sep;31(9):1825-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

