Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04JWS
|
|||
| Former ID |
DNC007823
|
|||
| Drug Name |
NSC-292213
|
|||
| Synonyms |
NSC-292213; NSC292213; CHEMBL222808; AC1L8B2I; NCIStruc2_001766; NCIStruc1_001644; ZINC1565922; NCI292213; BDBM50158387; NCGC00014649; CCG-37867; NCGC00014649-02; NCGC00097752-01; NCI60_002407; 4-[(3-carboxy-4-hydroxy-1-naphthyl)(oxo)acetyl]-1-hydroxy-2-naphthoic acid; 4-[2-(3-carboxy-4-hydroxy-naphthalen-1-yl)-2-oxo-acetyl]-1-hydroxy-naphthalene-2-carboxylic acid; 4-[2-(3-carboxy-4-hydroxynaphthalen-1-yl)-2-oxoacetyl]-1-hydroxynaphthalene-2-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
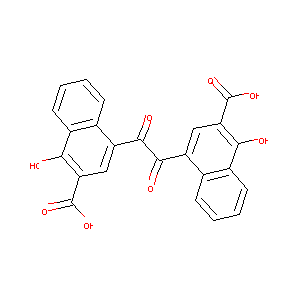 |
Download2D MOL |
||
| Formula |
C24H14O8
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=CC(=C2O)C(=O)O)C(=O)C(=O)C3=CC(=C(C4=CC=CC=C43)O)C(=O)O
|
|||
| InChI |
1S/C24H14O8/c25-19-13-7-3-1-5-11(13)15(9-17(19)23(29)30)21(27)22(28)16-10-18(24(31)32)20(26)14-8-4-2-6-12(14)16/h1-10,25-26H,(H,29,30)(H,31,32)
|
|||
| InChIKey |
BRCWHTNRFUVIQT-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 12-lipoxygenase (12-LOX) | Target Info | Inhibitor | [1] |
| Phosphoribosylaminoimidazolecarboxamide formyltransferase (ATIC) | Target Info | Inhibitor | [2] | |
| BioCyc | Lipoxin biosynthesis | |||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Arachidonic acid metabolism | |||
| Eicosanoid Synthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries. Bioorg Med Chem. 2007 Nov 15;15(22):6900-8. | |||
| REF 2 | Virtual screening of human 5-aminoimidazole-4-carboxamide ribonucleotide transformylase against the NCI diversity set by use of AutoDock to identif... J Med Chem. 2004 Dec 30;47(27):6681-90. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

