Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04CWI
|
|||
| Drug Name |
Galidesivir
|
|||
| Synonyms |
1EL1K52SH1; BCX 4430; BCX 4430 HCl; BCX4430; CS-3778; Galidesivir (hydrochloride); HY-18649; Immucillin-A; SB16943; SCHEMBL4838048; UNII-1EL1K52SH1
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 1 | [1] | |
| Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Preclinical | [2] | ||
| Severe acute respiratory syndrome (SARS) [ICD-11: 1D65] | Preclinical | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
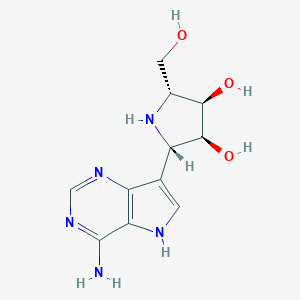 |
Download2D MOL |
||
| Formula |
C11H15N5O3
|
|||
| Canonical SMILES |
C1=C(C2=C(N1)C(=NC=N2)N)C3C(C(C(N3)CO)O)O
|
|||
| InChI |
1S/C11H15N5O3/c12-11-8-6(14-3-15-11)4(1-13-8)7-10(19)9(18)5(2-17)16-7/h1,3,5,7,9-10,13,16-19H,2H2,(H2,12,14,15)/t5-,7+,9-,10+/m1/s1
|
|||
| InChIKey |
AMFDITJFBUXZQN-KUBHLMPHSA-N
|
|||
| CAS Number |
CAS 249503-25-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | COVID-19 RNA-directed RNA polymerase (RdRp) | Target Info | Blocker | [3] |
| MERS-CoV RNA-directed RNA polymerase (RdRp) | Target Info | Inhibitor | [2] | |
| SARS-CoV RNA-directed RNA polymerase (RdRp) | Target Info | Inhibitor | [2] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03891420) A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19. U.S. National Institutes of Health. | |||
| REF 2 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||
| REF 3 | BioCryst Begins Clinical Trial with Galidesivir for Treatment of Patients with COVID-19 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

