Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03XQB
|
|||
| Former ID |
DNC010314
|
|||
| Drug Name |
(+/-)-7-fluoro-2-(4-methoxyphenyl)chroman-4-one
|
|||
| Synonyms |
CHEMBL598720; 844-66-6; 4h-1-benzopyran-4-one,7-fluoro-2,3-dihydro-2-(4-methoxyphenyl)-; 4H-1-Benzopyran-4-one, 7-fluoro-2,3-dihydro-2-(4-methoxyphenyl)-; AC1NRRFL; (+/-)-7-fluoro-2-(4-methoxyphenyl)chroman-4-one; CTK3D0370; DTXSID70414918; BDBM50310194; AKOS030553462; KB-306159; 7-fluoro-2-(4-methoxyphenyl)chroman-4-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
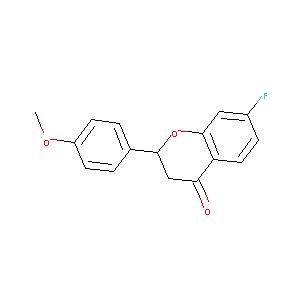 |
Download2D MOL |
||
| Formula |
C16H13FO3
|
|||
| Canonical SMILES |
COC1=CC=C(C=C1)C2CC(=O)C3=C(O2)C=C(C=C3)F
|
|||
| InChI |
1S/C16H13FO3/c1-19-12-5-2-10(3-6-12)15-9-14(18)13-7-4-11(17)8-16(13)20-15/h2-8,15H,9H2,1H3
|
|||
| InChIKey |
MQUZZZVJAVDIPR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 844-66-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Monoamine oxidase type B (MAO-B) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation via tryptamine | ||||
| Dopamine degradation | ||||
| Putrescine degradation III | ||||
| Noradrenaline and adrenaline degradation | ||||
| KEGG Pathway | Glycine, serine and threonine metabolism | |||
| Arginine and proline metabolism | ||||
| Histidine metabolism | ||||
| Tyrosine metabolism | ||||
| Phenylalanine metabolism | ||||
| Tryptophan metabolism | ||||
| Drug metabolism - cytochrome P450 | ||||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Dopaminergic synapse | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| 5-Hydroxytryptamine degredation | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| WikiPathways | Tryptophan metabolism | |||
| Dopamine metabolism | ||||
| Phase 1 - Functionalization of compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg Med Chem. 2010 Feb;18(3):1273-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

