Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03OSG
|
|||
| Former ID |
DCL000162
|
|||
| Drug Name |
MBX-8025
|
|||
| Synonyms |
Ppar; A agonist 2; SCHEMBL4950228; CHEMBL3545019; XJHXZGHPCAKRFK-UHFFFAOYSA-N; HY-100120; CS-0018099; {2-Methyl-4-[5-methyl-2-(4-trifluoromethyl-phenyl)-2H-[1,2,3]triazol-4-ylmethylsulfanyl]-phenoxy}-acetic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Obesity [ICD-11: 5B81; ICD-10: E66] | Phase 2/3 | [1] | |
| Familial hypercholesterolemia [ICD-11: 5C80.00; ICD-10: E78.0] | Phase 2 | [2] | ||
| Primary biliary cholangitis [ICD-11: DB96.1] | Phase 2 | [3] | ||
| Company |
Metabolex
|
|||
| Structure |
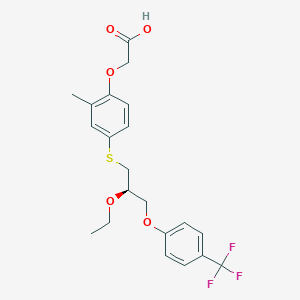 |
Download2D MOL |
||
| Formula |
C21H23F3O5S
|
|||
| Canonical SMILES |
CCOC(COC1=CC=C(C=C1)C(F)(F)F)CSC2=CC(=C(C=C2)OCC(=O)O)C
|
|||
| InChI |
1S/C21H23F3O5S/c1-3-27-17(11-28-16-6-4-15(5-7-16)21(22,23)24)13-30-18-8-9-19(14(2)10-18)29-12-20(25)26/h4-10,17H,3,11-13H2,1-2H3,(H,25,26)/t17-/m1/s1
|
|||
| InChIKey |
JWHYSEDOYMYMNM-QGZVFWFLSA-N
|
|||
| CAS Number |
CAS 851528-79-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Peroxisome proliferator-activated receptor delta (PPARD) | Target Info | Agonist | [4] |
| KEGG Pathway | PPAR signaling pathway | |||
| Wnt signaling pathway | ||||
| Pathways in cancer | ||||
| Acute myeloid leukemia | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | Wnt signaling pathway | |||
| Pathway Interaction Database | Presenilin action in Notch and Wnt signaling | |||
| RXR and RAR heterodimerization with other nuclear receptor | ||||
| Reactome | Import of palmitoyl-CoA into the mitochondrial matrix | |||
| Regulation of pyruvate dehydrogenase (PDH) complex | ||||
| Nuclear Receptor transcription pathway | ||||
| WikiPathways | Wnt Signaling Pathway and Pluripotency | |||
| Nuclear Receptors in Lipid Metabolism and Toxicity | ||||
| NRF2 pathway | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Vitamin D Receptor Pathway | ||||
| Ectoderm Differentiation | ||||
| Adipogenesis | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024671) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | ClinicalTrials.gov (NCT00701883) Safety and Benefit of MBX-8025 With and Without Commonly Used Statins in Moderately Overweight Patients With High Cholesterol. U. S. National Institute of Health. 2008. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

