Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02NXM
|
|||
| Former ID |
DNCL002561
|
|||
| Drug Name |
GSK2110183
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | leukaemia [ICD-11: 2A60-2B33; ICD-9: 208.9] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199] | Phase 2 | [2] | ||
| Multiple myeloma [ICD-11: 2A83; ICD-10: C90.0] | Phase 1 | [3], [4] | ||
| Company |
GlaxoSmithKline
|
|||
| Structure |
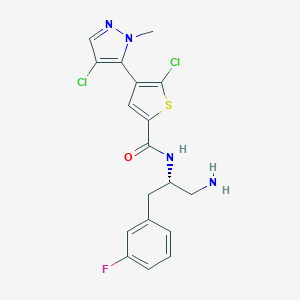 |
Download2D MOL |
||
| Formula |
C18H17Cl2FN4OS
|
|||
| Canonical SMILES |
CN1C(=C(C=N1)Cl)C2=C(SC(=C2)C(=O)NC(CC3=CC(=CC=C3)F)CN)Cl
|
|||
| InChI |
1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1
|
|||
| InChIKey |
AFJRDFWMXUECEW-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 1047644-62-1
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:131168
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01445587) A Study of GSK2110183 in Subjects With Proteasome Inhibitor Refractory Multiple Myeloma. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT01531894) Continuation Study of the Oral AKT Inhibitor GSK2110183. U.S. National Institutes of Health. | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7890). | |||
| REF 4 | ClinicalTrials.gov (NCT02040480) Bioavailability and Food Effect Study of Gelatin Formulation and Immediate Release Tablet Formulation of Afuresertib. U.S. National Institutes of Health. | |||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1479). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

