Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01UBX
|
|||
| Former ID |
DAP001342
|
|||
| Drug Name |
Aclarubicin
|
|||
| Synonyms |
Aclacin; Aclacur; Aclarubicine; Aclarubicino; Aclarubicinum; Jaclacin; Aclacinomycin A; Aclucinomycin A; Antibiotic MA 144A; Antibiotic MA 144A1; Antibiotic MA 144G1; MA 144G1; Aclarubicine [INN-French]; Aclarubicino [INN-Spanish]; Aclarubicinum [INN-Latin]; Antibiotic MA144-A1; MA 144-A1; MA-144A1; Acene-1-carboxylate; Aclarubicin (USAN/INN); Aclarubicin [USAN:BAN:INN]; Alpha-L-lyxo-hexopyranosyl]-oxy]-1-naphthacenecarboxalic acid methyl ester; 10-epi-Aclacinomycin A
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
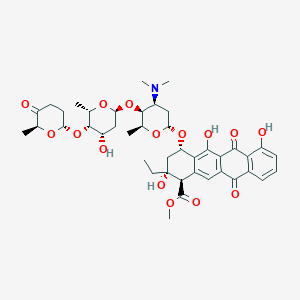 |
Download2D MOL
|
||
| Formula |
C42H53NO15
|
|||
| Canonical SMILES |
CCC1(CC(C2=C(C3=C(C=C2C1C(=O)OC)C(=O)C4=C(C3=O)C(=CC=C4)O)O)OC5CC(C(C(O5)C)OC6CC(C(C(O6)C)OC7CCC(=O)C(O7)C)O)N(C)C)O
|
|||
| InChI |
1S/C42H53NO15/c1-8-42(51)17-28(33-22(35(42)41(50)52-7)14-23-34(38(33)49)37(48)32-21(36(23)47)10-9-11-26(32)45)56-30-15-24(43(5)6)39(19(3)54-30)58-31-16-27(46)40(20(4)55-31)57-29-13-12-25(44)18(2)53-29/h9-11,14,18-20,24,27-31,35,39-40,45-46,49,51H,8,12-13,15-17H2,1-7H3/t18-,19-,20-,24-,27-,28-,29-,30-,31-,35-,39+,40+,42+/m0/s1
|
|||
| InChIKey |
USZYSDMBJDPRIF-SVEJIMAYSA-N
|
|||
| CAS Number |
CAS 57576-44-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:77980
|
|||
| SuperDrug ATC ID |
L01DB04
|
|||
| SuperDrug CAS ID |
cas=057576440
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Inhibitor | [3], [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Linker length in podophyllotoxin-acridine conjugates determines potency in vivo and in vitro as well as specificity against MDR cell lines. Anticancer Drug Des. 2001 Dec;16(6):305-15. | |||
| REF 4 | 1,3-Dihydroxyacridone derivatives as inhibitors of herpes virus replication. Antiviral Res. 2000 Feb;45(2):123-34. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

