Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01FGR
|
|||
| Former ID |
DAP000490
|
|||
| Drug Name |
Diphenhydramine
|
|||
| Synonyms |
diphenhydramine; 58-73-1; Benadryl; Benzhydramine; Probedryl; Alledryl; Antistominum; Dihidral; 2-(Benzhydryloxy)-N,N-dimethylethanamine; Benzhydraminum; Benzhydroamina; Diphantine; Difenhydramin; Ibiodral; Difedryl; Dibondrin; Mephadryl; Desentol; Dermistina; Betramin; Benzhydril; Benzantine; Benachlor; Bagodryl; Syntedril; Novamina; Medidryl; Hyadrine; Histaxin; Etanautine; Drylistan; Diabylen; Diabenyl; Dermodrin; Benodine; Baramine; Allergical; Debendrin; Benadrin; Dabylen; Antomin; Nausen; Benapon; Dylamon; Benylin; Benylan; Benodin; Amidryl; Aleryl; Allerdryl; Allergeval; Allergina; Allergival; Antitussive; Automin; Banophen; Beldin; Belix; Bena; Benhydramin; Benzhydryl; Compoz; Dibendrin; Dibenil; Difenhidramina; Difenidramina; Dimedrol; Dimedryl; Dimehydrinate; Diphen; Diphenhist; Diphenhydraminum; Diphenylhydramin; Diphenylhydramine; Dryistan; Genahist; Hydramine; Hyrexin; Restamin; Rigidil; Siladryl; Silphen; Syntodril; Allergan B; Allermax Caplets; BENADRYL HCl; Banophen Caplets; Benadryl Allergy; Difenidramina [Italian]; Dimedrol base; Diphen Cough; Diphenhist Captabs; Diphenhydramine Base; Diphenhydramine HCl; Nytol Quickcaps; Nytol Quickgels;Twilite Caplets; Unisom Sleepgels Maximum Strength; Dormarex 2; FAR 90X2; PM 255; S51; Aller-Med; Ben-allergin; Benadryl (TN); Benadryl (hydrochloride); Beta-Dimethylaminoethanol diphenylmethyl ether; Beta-Dimethylaminoethylbenzhydrylether; Beta-dimethylaminoethyl benzhydryl ether; Difenhidramina [INN-Spanish]; Dimedrol (TN); Diphenhydraminum [INN-Latin]; Nervine Nighttime Sleep-Aid; Nytol (TN); O-Benzhydryldimethylaminoethanol; Restamin (TN); Sleep-Eze D; Sleep-Eze D Extra Strength; DIPHENHYDRAMINE, ANTISTOMINUM, BENZHYDRAMINE; Diphenhydramine (JP15/INN); Diphenhydramine [INN:BAN:JAN]; Alpha-(2-Dimethylaminoethoxy)diphenylmethane; N-(Benzhydryloksy-etylo)dwumetyloamina; N-(Benzhydryloksy-etylo)dwumetyloamina [Polish]; Beta-Dimethylamino-aethyl-benzhydryl-aether; Beta-Dimethylamino-aethyl-benzhydryl-aether [German]; N,N-Dimethyl-2-(diphenylmethoxy)-ethylamine hydrochloride; N-[2-(BENZHYDRYLOXY)ETHYL]-N,N-DIMETHYLAMINE; N-(2-(Diphenylmethoxy)ethyl)-N,N-dimethylamine; 2-(Benzhydryloxy)-N,N-dimethylethylamine; 2-(Benzhydryloxy)-N,N-dimethylethylamine, hydrochloride; 2-(Diphenylmethoxy)-N,N-dimethylethylamine; 2-(diphenylmethoxy)-N,N-dimethylethanamine; 2-Diphenylmethoxy-N,N-dimethylethylamine; 2-[(diphenylmethyl)oxy]-N,N-dimethylethanamine; 2-benzhydryloxy-N,N-dimethylethanamine; 2-benzhydryloxyethyl-N,N-dimethylammonium; 2-diphenylmethoxy-N,N-demthylethanamine; 2PM
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Meniere disease [ICD-11: AB31.0; ICD-10: H81] | Approved | [1] | |
| Therapeutic Class |
Antiallergic Agents
|
|||
| Company |
Johnson & Johnson
|
|||
| Structure |
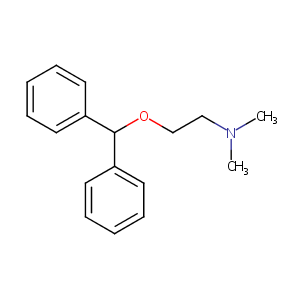 |
Download2D MOL |
||
| Formula |
C17H21NO
|
|||
| Canonical SMILES |
CN(C)CCOC(C1=CC=CC=C1)C2=CC=CC=C2
|
|||
| InChI |
1S/C17H21NO/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,17H,13-14H2,1-2H3
|
|||
| InChIKey |
ZZVUWRFHKOJYTH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 58-73-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9174, 511392, 608048, 3154507, 5021578, 7847366, 7849634, 7885212, 7979103, 8151972, 10508422, 10528411, 10544720, 11111059, 11111060, 11335543, 11360782, 11363874, 11366436, 11368998, 11371539, 11374238, 11377160, 11407308, 11427137, 11461754, 11466093, 11467213, 11484697, 11485906, 11488777, 11490216, 11492335, 11494794, 14749996, 25768169, 26713829, 26751623, 29222243, 46505484, 47440177, 47588927, 47810670, 47810671, 48035032, 48110379, 48110380, 48184925, 48184926, 48415909
|
|||
| ChEBI ID |
CHEBI:4636
|
|||
| ADReCS Drug ID | BADD_D00686 ; BADD_D00687 | |||
| SuperDrug ATC ID |
D04AA32; R06AA02
|
|||
| SuperDrug CAS ID |
cas=000058731
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [2], [3] |
| Histamine N-methyltransferase (HNMT) | Target Info | Inhibitor | [4] | |
| BioCyc | Histamine degradation | |||
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Histidine metabolism | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Pathwhiz Pathway | Histidine Metabolism | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| Methylation Pathways | ||||
| Metapathway biotransformation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Intact cell binding for in vitro prediction of sedative and non-sedative histamine H1-receptor antagonists based on receptor internalization. J Pharmacol Sci. 2008 May;107(1):66-79. | |||
| REF 3 | Histamine as an autocrine regulator of leukemic cell proliferation. Leuk Lymphoma. 2000 Jan;36(3-4):367-73. | |||
| REF 4 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

