Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00NVM
|
|||
| Former ID |
DCL000995
|
|||
| Drug Name |
SNS-595
|
|||
| Synonyms |
Voreloxin; Vosaroxin; AG 7352; SNS 595; SPC 595; AG 7352 (TN); AG-7352; SNS 595 (TN); SPC 595 (TN); SPC-595; Voreloxin (TN); Voreloxin (USAN); SNS-595 (TN); 1,4-Dihydro-7-(3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Phase 3 | [1] | |
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 2 | [2] | ||
| Company |
Sunesis Pharmaceuticals Inc
|
|||
| Structure |
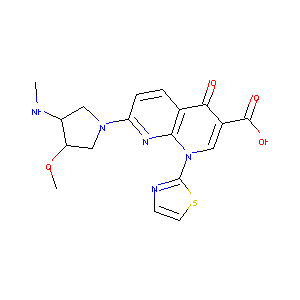 |
Download2D MOL |
||
| Formula |
C18H19N5O4S
|
|||
| Canonical SMILES |
CNC1CN(CC1OC)C2=NC3=C(C=C2)C(=O)C(=CN3C4=NC=CS4)C(=O)O
|
|||
| InChI |
1S/C18H19N5O4S/c1-19-12-8-22(9-13(12)27-2)14-4-3-10-15(24)11(17(25)26)7-23(16(10)21-14)18-20-5-6-28-18/h3-7,12-13,19H,8-9H2,1-2H3,(H,25,26)/t12-,13-/m0/s1
|
|||
| InChIKey |
XZAFZXJXZHRNAQ-STQMWFEESA-N
|
|||
| CAS Number |
CAS 175414-77-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II (TOP2) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01191801) Study of Vosaroxin or Placebo in Combination With Cytarabine in Patients With First Relapsed or Refractory AML (VALOR). U.S. National Institutes of Health. | |||
| REF 2 | Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2008 Sep;13(3):523-36. | |||
| REF 3 | A phase 1b/2 study of vosaroxin in combination with cytarabine in patients with relapsed or refractory acute myeloid leukemia.Haematologica.2015 Feb;100(2):231-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

