Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M0CF
|
|||
| Former ID |
DNC003203
|
|||
| Drug Name |
Farnesol
|
|||
| Synonyms |
farnesol; 4602-84-0; Farnesyl alcohol; 3,7,11-trimethyldodeca-2,6,10-trien-1-ol; Spectrum_001282; 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (2Z,6Z)-; ACMC-20aplp; ACMC-209ukw; SpecPlus_000549; AC1L1FOK; Spectrum3_001070; Spectrum2_001397; Spectrum4_001221; KBioSS_001762; KBioGR_001682; 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (2E,6Z)-; DivK1c_006645; SPBio_001414; GTPL3215; DTXSID3032389; KBio2_006898; KBio1_001589; KBio3_001880; KBio2_004330; CTK1B4647; CTK1D5922; KBio2_001762; CTK0H6624; CTK0E6577; MolPort-006-116-336
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
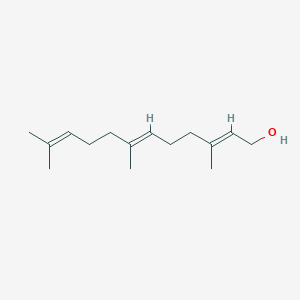 |
Download2D MOL |
||
| Formula |
C15H26O
|
|||
| Canonical SMILES |
CC(=CCCC(=CCCC(=CCO)C)C)C
|
|||
| InChI |
1S/C15H26O/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-16/h7,9,11,16H,5-6,8,10,12H2,1-4H3/b14-9+,15-11+
|
|||
| InChIKey |
CRDAMVZIKSXKFV-YFVJMOTDSA-N
|
|||
| CAS Number |
CAS 4602-84-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5276903, 8152113, 11341820, 11362003, 11364176, 11366738, 11369300, 11371979, 11374820, 11377462, 11409038, 11485150, 11487405, 11489213, 11490885, 11493035, 11495096, 29222462, 77162380, 85086019, 104303185, 125337280, 125370895, 125948325, 126675270, 127461390, 127665825, 135651486, 160811400, 162300594, 162308493, 162450832, 162508624, 162528815, 163305392, 166223302, 176255878, 221672928, 223808186, 252140091, 252258905, 252766539
|
|||
| ChEBI ID |
CHEBI:16619
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Monoamine oxidase type B (MAO-B) | Target Info | Inhibitor | [2] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation via tryptamine | ||||
| Dopamine degradation | ||||
| Putrescine degradation III | ||||
| Noradrenaline and adrenaline degradation | ||||
| KEGG Pathway | Glycine, serine and threonine metabolism | |||
| Arginine and proline metabolism | ||||
| Histidine metabolism | ||||
| Tyrosine metabolism | ||||
| Phenylalanine metabolism | ||||
| Tryptophan metabolism | ||||
| Drug metabolism - cytochrome P450 | ||||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Dopaminergic synapse | ||||
| Cocaine addiction | ||||
| Amphetamine addiction | ||||
| Alcoholism | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| 5-Hydroxytryptamine degredation | ||||
| Dopamine receptor mediated signaling pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | |||
| WikiPathways | Tryptophan metabolism | |||
| Dopamine metabolism | ||||
| Phase 1 - Functionalization of compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3215). | |||
| REF 2 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

