Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07OZR
|
|||
| Former ID |
DCL000248
|
|||
| Drug Name |
TKI258
|
|||
| Synonyms |
Dovitinib; 405169-16-6; CHIR-258; TKI-258; Chir 258; 4-Amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one; CHIR258; Dovitinib (TKI-258, CHIR-258); UNII-I35H55G906; CHEMBL522892; 804551-71-1; I35H55G906; TKI 258; 1027263-12-2; (3Z)-4-Amino-5-fluoro-3-[5-(4-methyl-1-piperazinyl)-1,3-dihydro-2H-benzimidazol-2-ylidene]-2(3H)-quinolinone; C21H21FN6O; 4-Amino-5-fluoro-3-(5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl)quinolin-2(1H)-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Renal cell carcinoma [ICD-11: 2C90; ICD-10: C64; ICD-9: 189] | Phase 3 | [1] | |
| Endometrial cancer [ICD-11: 2C76] | Phase 2 | [2] | ||
| Triple negative breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [3] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Novartis & Chiron Corp
|
|||
| Structure |
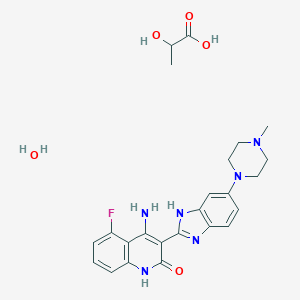 |
Download2D MOL
|
||
| Formula |
C24H29FN6O5
|
|||
| Canonical SMILES |
CC(C(=O)O)O.CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=C(C5=C(C=CC=C5F)NC4=O)N.O
|
|||
| InChI |
1S/C21H21FN6O.C3H6O3.H2O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29;1-2(4)3(5)6;/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29);2,4H,1H3,(H,5,6);1H2
|
|||
| InChIKey |
QDPVYZNVVQQULH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 915769-50-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14854259, 16526641, 16823163, 39896483, 45633748, 50100092, 56374260, 74418884, 109693511, 121280185, 135261170, 136367994, 141243978, 141243980, 141913081, 141913174, 144115820, 152258853, 152344214, 160647703, 162205181, 164044350, 174529303, 177748727, 178102585, 180190845, 188899516, 196378498, 198975799, 223397268, 226534136, 235322191, 249565565, 249565566, 249565567, 249582865, 251856461, 252215131, 252543299
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fibroblast growth factor receptor 3 (FGFR3) | Target Info | Inhibitor | [4] |
| Platelet-derived growth factor receptor (PDGFR) | Target Info | Inhibitor | [4] | |
| KEGG Pathway | MAPK signaling pathway | |||
| Ras signaling pathway | ||||
| Rap1 signaling pathway | ||||
| Endocytosis | ||||
| PI3K-Akt signaling pathway | ||||
| Signaling pathways regulating pluripotency of stem cells | ||||
| Regulation of actin cytoskeleton | ||||
| Pathways in cancer | ||||
| MicroRNAs in cancer | ||||
| Bladder cancer | ||||
| Central carbon metabolism in cancer | ||||
| Panther Pathway | FGF signaling pathway | |||
| Reactome | FGFR3 mutant receptor activation | |||
| WikiPathways | Regulation of Actin Cytoskeleton | |||
| Endochondral Ossification | ||||
| Bladder Cancer | ||||
| Neural Crest Differentiation | ||||
| Signaling by FGFR | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01223027) Study of Dovitinib Versus Sorafenib in Patients With Metastatic Renal Cell Carcinoma. U.S. National Institutes of Health. | |||
| REF 2 | Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer. 2015 Aug 1;121(15):2612-7. | |||
| REF 3 | ClinicalTrials.gov (NCT01262027) TKI258 for Metastatic Inflammatory Breast Cancer Patients. U.S. National Institutes of Health. | |||
| REF 4 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

