Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06RGG
|
|||
| Former ID |
DAP000083
|
|||
| Drug Name |
Levothyroxine
|
|||
| Synonyms |
Eltroxin; Euthyrox; Forthyron; Laevothyroxinum; Laevoxin; Levolet; Levothroid; Levothyrox; Levothyroxin; Levoxyl; Oroxine; Synthroid; THX; Tetraiodothyronine; Thyratabs; Thyrax; Thyreoideum; Thyroxevan; Thyroxin; Thyroxinal; Levothyroxine sodium; Synthroid sodium; Thyroxine [BAN]; Thyroxine iodine; LT00440967; T4 levothyroxine; DL-Thyroxin; Eltroxin (TN); Euthyrox (TN); Eutirox (TN); Forthyron (TN); L-Thryoxin; L-Thyroxin; L-thyroxine; Laevothyroxinum (acid); Levaxin (TN); Levo-T; Levothyroxine (BAN); Levothyroxinum (acid); Levoxyl (TN); Synthroid (TN); T-3850; T4 (Hormone); Thyrax (TN); Thyrox (TN); Thyroxine (VAN); Thyroxine (l); L-thyroxine (TN); Levothyroxine Sodium (L-thyroxine); Levothroid (*Sodiumsalt*); Synthroid (*Sodium salt*); Thyroxine, L-(8CI); L-3,5,3',5'-Tetraiodothyronine; O-(4-Hydroxy-3,5-diiodophenyl)-3,5-diiodotyrosine; Beta-((3,5-Diiodo-4-hydroxyphenoxy)-3,5-diiodophenyl)alanine; O-(4-Hydroxy-3,5-diidophenyl)-3,5-diiodo-L-tyrosine; O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine; (-)-Thyroxine; (125I)T4; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid; 2-amino-3-[4-(4-hydroxy-3,5-diiodo-phenoxy)-3,5-diiodo-phenyl]-propanoic acid; 3,3',5,5"-Tetraiodo-L-thyronine; 3,3',5,5''-Tetraiodo-L-thyronine; 3,3',5,5'-Tetraiodo-L-thyronine; 3,5,3',5'-Tetraiodo-L-thyronine; 3,5,3',5'-Tetraiodothyronine; 3,5,3'5'-Tetraiodo-L-thyronine; 3-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)alanine; 3-[4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-L-alanine; 4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodo-L-phenylalanine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypothyroidism [ICD-11: 5A00; ICD-10: E03.8, E03.9; ICD-9: 244] | Approved | [1], [2] | |
| Therapeutic Class |
Antithyroid Agents
|
|||
| Company |
Abbott Laboratories
|
|||
| Structure |
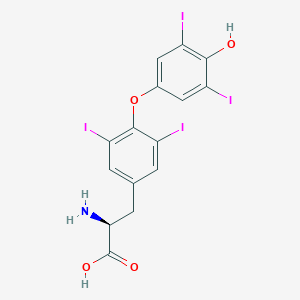 |
Download2D MOL |
||
| Formula |
C15H11I4NO4
|
|||
| Canonical SMILES |
C1=C(C=C(C(=C1I)OC2=CC(=C(C(=C2)I)O)I)I)CC(C(=O)O)N
|
|||
| InChI |
1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1
|
|||
| InChIKey |
XUIIKFGFIJCVMT-LBPRGKRZSA-N
|
|||
| CAS Number |
CAS 51-48-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:18332
|
|||
| ADReCS Drug ID | BADD_D01285 ; BADD_D01286 | |||
| SuperDrug ATC ID |
H03AA01
|
|||
| SuperDrug CAS ID |
cas=000055038
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Actinomycetales | ||||
|
Studied Microbe: Actinomyces
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Human | Experimental Sample | Faeces | |
| Disease or Condition | Inflammatory bowel disease | |||
| Description | The abundance of Actinomyces was increased by Levothyroxine . | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Eubacterium eligens
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium eligens was decreased by Thyroxine (L) (adjusted p-values: 1.70E-03). | |||
|
Studied Microbe: Lacrimispora saccharolytica
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Lacrimispora saccharolytica was decreased by Thyroxine (L) (adjusted p-values: 1.27E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Lactobacillus paracasei
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Lactobacillus paracasei was decreased by Thyroxine (L) (adjusted p-values: 9.36E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Thyroid hormone receptor alpha (THRA) | Target Info | Antagonist | [5], [6] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Thyroid hormone signaling pathway | ||||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | |||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | Endochondral Ossification | |||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4627). | |||
| REF 2 | Current methodology to assess bioequivalence of levothyroxine sodium products is inadequate. AAPS J. 2005 Mar 30;7(1):E42-6. | |||
| REF 3 | Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020 Jan 17;11(1):362. | |||
| REF 4 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 5 | Thyroid hormone resistance and pituitary enlargement after thyroid ablation in a woman on levothyroxine treatment. Thyroid. 2008 Oct;18(10):1119-23. | |||
| REF 6 | Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007 Mar;3(3):249-59. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

