Target expression details
| Target General Information | |||||
|---|---|---|---|---|---|
| Target ID | T06671 | ||||
| Target Name | Interleukin-18 | ||||
| Synonyms | IFN-gamma-inducing factor; IL-1 gamma; IL-18; Interferon-gamma inducing factor; Interleukin-1 gamma; IL18 | ||||
| Target Type | Clinical Trial | ||||
| Gene Name | IL18 | ||||
| Biochemical Class | Cytokine: interleukin | ||||
| UniProt ID | IL18_HUMAN | ||||
| Target Gene Expression Profiles in the Disease-Relevant Drug Targeted Tissue of the Patients and Healthy Individuals | |||||
| Disease | Type 2 diabetes | ||||
| Example drug | GSK-1070806 | Phase 2 | [1], [2] | ||
| Tissue | Liver tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.12 Z-score: 1.81 P-value: 1.46E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | MEDI-2338 | Phase 1 | [3], [2] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.14 Z-score: -0.31 P-value: 5.59E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
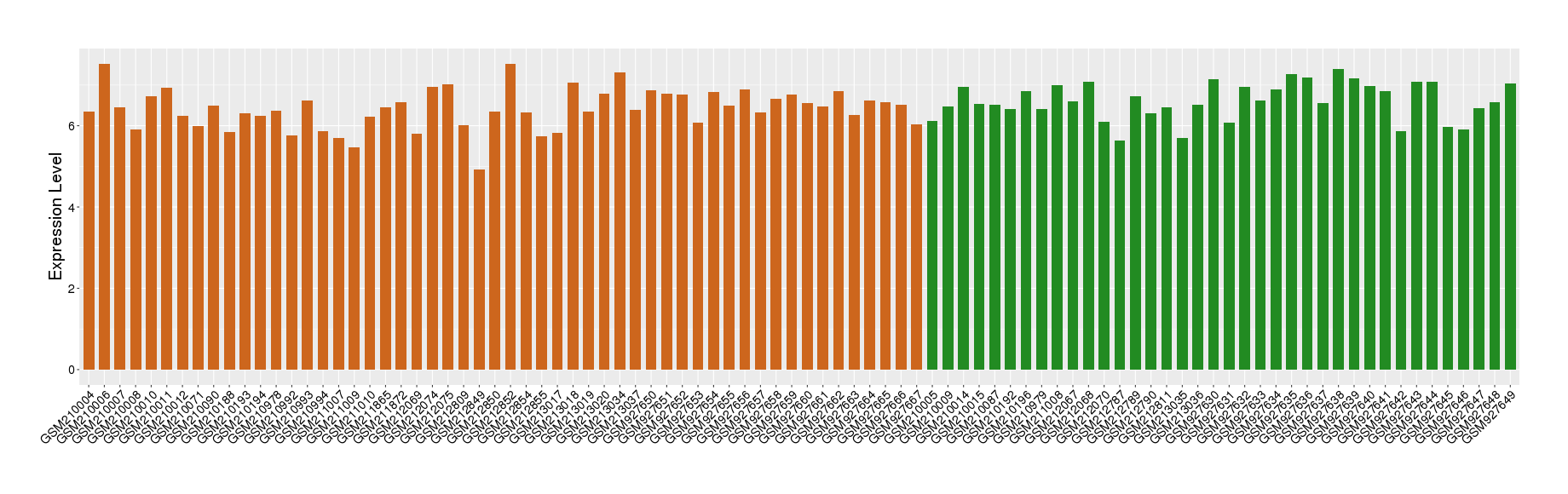
|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | MEDI-2338 | Phase 1 | [3], [2] | ||
| Tissue | Small airway epithelium | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: -0.10 Z-score: -0.21 P-value: 6.04E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Ovarian cancer | ||||
| Example drug | Iboctadekin + Doxil | Phase 1 | [4], [5], [2] | ||
| Tissue | Ovarian tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 1.05 Z-score: 1.26 P-value: 9.80E-04 |
||||
| Level of differential expression between the patients in the disease section of the tissue and the patients in the normal section of the tissue adjacent to the disease section | Fold-change: -0.58 Z-score: -0.29 P-value: 4.18E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Rheumatoid arthritis | ||||
| Example drug | IL-18BP | Discontinued in Phase 1 | [6], [2] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual | Fold-change: 0.15 Z-score: 0.25 P-value: 8.24E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
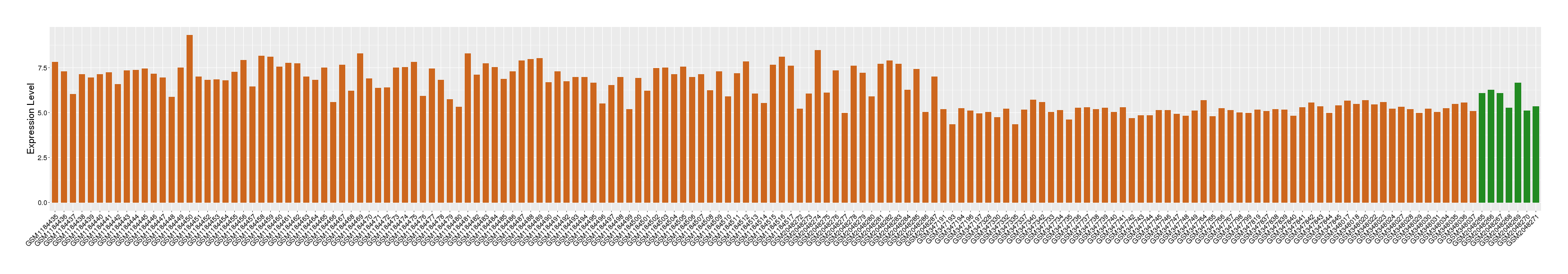
|
|||||
| Target Gene Expression Profiles in Other Tissues of Healthy Individuals | |||||
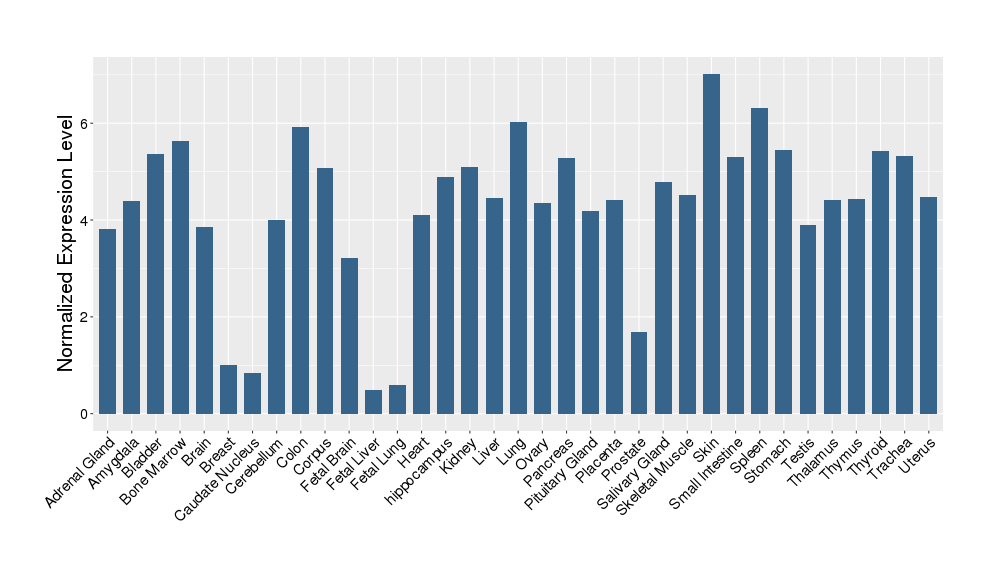
|
|||||
| Reference | |||||
| REF 1 | ClinicalTrials.gov (NCT01648153) Investigate the Efficacy and Safety of GSK1070806 in Obese Subjects With T2DM. U.S. National Institutes of Health. | ||||
| REF 2 | NCBI GEO: archive for functional genomics data sets--update. | ||||
| REF 3 | ClinicalTrials.gov (NCT01322594) A Study to Evaluate the Safety of MEDI2338 in Subjects With Chronic Obstructive Pulmonary Disease. U.S. National Institutes of Health. | ||||
| REF 4 | ClinicalTrials.gov (NCT00659178) Combination Study Of SB-485232 (Interleukin 18) And Doxil For Advanced Stage Epithelial Ovarian Cancer. U.S. National Institutes of Health. | ||||
| REF 5 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | ||||
| REF 6 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013227) | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

