Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O5GK
|
||||
| Former ID |
DNCL001979
|
||||
| Drug Name |
R-phenserine
|
||||
| Synonyms |
Posiphen (TN)
|
||||
| Indication | Alzheimer disease; Mild cognitive impairment [ICD9: 331.0, 331.83; ICD10:F06.7, G30, G31.84] | Phase 2 | [1] | ||
| Company |
QR Pharma
|
||||
| Structure |
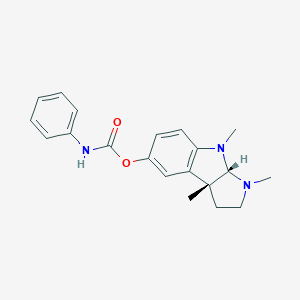
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| WikiPathways | Monoamine Transport | ||||
| Biogenic Amine Synthesis | |||||
| Acetylcholine Synthesis | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021506) | ||||
| REF 2 | An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Curr Alzheimer Res. 2005 Jul;2(3):281-90. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

