Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M5VV
|
||||
| Former ID |
DIB003532
|
||||
| Drug Name |
177Lu-DOTATATE
|
||||
| Indication | HCV infection [ICD9: 070.4, 070.5, 070.70; ICD10:B17.1, B18.2] | Phase 2 | [524302] | ||
| Company |
Advanced accelerator applications
|
||||
| Structure |
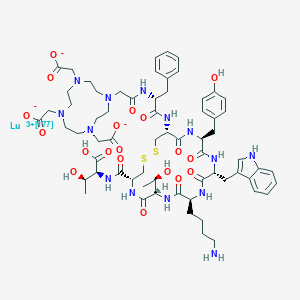
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Somatostatin receptor | Target Info | Modulator | [532708] | |
| Somatostatin receptor type 4 | Target Info | Inhibitor | [1725888] | ||
| References | |||||
| Ref 532708 | Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014 May;43(4):518-25. | ||||
| Ref 1725888 | New Drug in Development Shows Improved Progression-Free Survival for Patients with Advanced Metastatic Midgut Neuroendocrine Tumors | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

