Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04AMD
|
||||
| Former ID |
DIB013123
|
||||
| Drug Name |
ZD-6126
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Phase 2 | [521567] | ||
| Company |
Angiogene Pharmaceuticals Ltd; Angiogene
|
||||
| Structure |
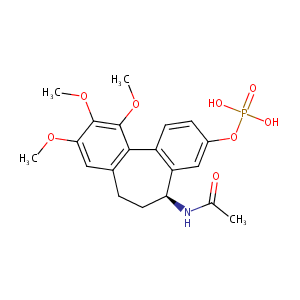
|
Download2D MOL |
|||
| Formula |
C20H24NO8P
|
||||
| CAS Number |
CAS 219923-05-4
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Tubulin beta | Target Info | Inhibitor | [529270] | |
| PANTHER Pathway | Cytoskeletal regulation by Rho GTPase | ||||
| Huntington disease | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

