Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D09LMK
|
||||
| Former ID |
DIB001549
|
||||
| Drug Name |
Lu-AA21004
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Mood disorder [ICD10:F30-F39] | Phase 3 | [1] | ||
| Company |
Lundbeck; takeda pharmaceuticals
|
||||
| Structure |
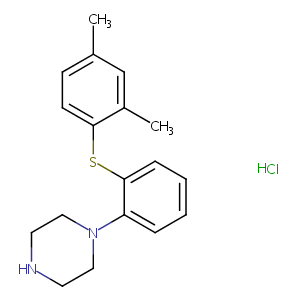
|
Download2D MOL |
|||
| Formula |
C18H22N2S
|
||||
| Canonical SMILES |
c1(ccc(c(c1)C)Sc1ccccc1N1CCNCC1)C.Cl
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Sodium-dependent serotonin transporter | Target Info | Modulator | [2], [1], [3] | |
| KEGG Pathway | Serotonergic synapse | ||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | 5HT1 type receptor mediated signaling pathway | ||||
| 5HT2 type receptor mediated signaling pathway | |||||
| 5HT3 type receptor mediated signaling pathway | |||||
| 5HT4 type receptor mediated signaling pathway | |||||
| WikiPathways | Monoamine Transport | ||||
| SIDS Susceptibility Pathways | |||||
| NRF2 pathway | |||||
| Synaptic Vesicle Pathway | |||||
| Serotonin Transporter Activity | |||||
| References | |||||
| REF 1 | Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav. 2013 Apr;105:41-50. | ||||
| REF 2 | A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012 Jun;15(5):589-600. | ||||
| REF 3 | 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85-9. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

