Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04TQT
|
||||
| Former ID |
DIB014463
|
||||
| Drug Name |
Barusiban
|
||||
| Synonyms |
FE-200440
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Androgen decline [ICD10:E20-E35] | Phase 2 | [521712] | ||
| Company |
Ferring Pharmaceuticals Inc
|
||||
| Structure |
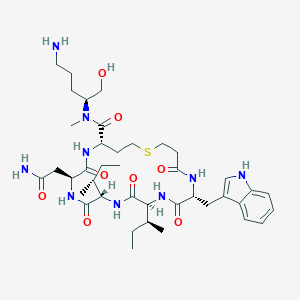
|
Download2D MOL |
|||
| Formula |
C40H63N9O8S
|
||||
| Canonical SMILES |
N1C(=O)[C@@H](NC(=O)[C@H](NC(=O)CCSCC[C@H](NC(=O)[C@@H]<br />(NC(=O)[C@@H]1[C@@H](CC)C)CC(=O)N)C(=O)N([C@H](CO)CCCN)<br />C)Cc1c[nH]c2c1cccc2)[C@H](CC)C
|
||||
| CAS Number |
CAS 285571-64-4
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Oxytocin receptor | Target Info | Antagonist | [530031] | |
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| PANTHER Pathway | Oxytocin receptor mediated signaling pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

