Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02ZUO
|
||||
| Former ID |
DCL000117
|
||||
| Drug Name |
Forodesine
|
||||
| Synonyms |
Fodosine; IMH; Immucillin H; Fodosine (TN); Immucillin-H; Forodesine (USAN/INN); (1S)-1,4-dideoxy-4-imino-(9-deazahypoxanthin-9-yl)-D-ribitol; (2R,3R,4S,5S)-2-(hydroxymethyl)-5-(4-hydroxy-5H-pyrrolo[3,2-d]pyrimidin-7-yl)pyrrolidine-3,4-diol; 1,4-dideoxy-4-aza-1-(s)-(9-deazahypoxanthin-9-yl)-d-ribitol; 1-(9-deazahypoxanthin-9-yl)-1,4-dideoxy-1,4-iminoribitol; 7-[(2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl]-1,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one; 7-[(2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)pyrrolidin-2-yl]-1,5-dihydropyrrolo[3,2-d]pyrimidin-4-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
BioCryst Pharma.
|
||||
| Structure |
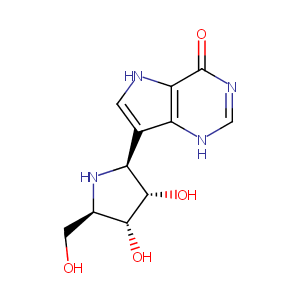
|
Download2D MOL |
|||
| Formula |
C11H14N4O4
|
||||
| InChI |
InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1
|
||||
| InChIKey |
IWKXDMQDITUYRK-KUBHLMPHSA-N
|
||||
| CAS Number |
CAS 209799-67-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
820339, 824927, 829471, 854455, 854458, 7888374, 11532495, 14774821, 15392822, 24770619, 24770621, 24770665, 36887500, 46391523, 46391948, 46392657, 46394112, 46394113, 46530883, 47208247, 49689710, 57404625, 77434485, 85856193, 103500429, 104077585, 104632275, 117682608, 134338803, 134339052, 134340118, 135020342, 135675084, 137042995, 141755560, 144080329, 160644592, 160779316, 165245606, 180372225, 186024301, 198952847, 224718993, 226820362, 241051921, 243906436, 252166484
|
||||
| ChEBI ID |
ChEBI:43362
|
||||
| Target and Pathway | |||||
| Target(s) | Purine nucleoside phosphorylase | Target Info | Inhibitor | [536974], [537256], [537497] | |
| BioCyc Pathway | Arsenate detoxification I (glutaredoxin) | ||||
| Purine nucleotides degradation | |||||
| Urate biosynthesis/inosine 5'-phosphate degradation | |||||
| Purine deoxyribonucleosides degradation | |||||
| Purine ribonucleosides degradation to ribose-1-phosphate | |||||
| Guanosine nucleotides degradation | |||||
| Adenosine nucleotides degradation | |||||
| Superpathway of purine nucleotide salvage | |||||
| Adenine and adenosine salvage III | |||||
| Guanine and guanosine salvage | |||||
| NetPath Pathway | TCR Signaling Pathway | ||||
| EGFR1 Signaling Pathway | |||||
| PathWhiz Pathway | Purine Metabolism | ||||
| Nicotinate and Nicotinamide Metabolism | |||||
| Reactome | Purine salvage | ||||
| Purine catabolism | |||||
| References | |||||
| Ref 536739 | Emerging drugs in cutaneous T cell lymphoma. Expert Opin Emerg Drugs. 2008 Jun;13(2):345-61. | ||||
| Ref 543027 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8272). | ||||
| Ref 536974 | Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma. 2008 Dec;8 Suppl 5:S187-92. | ||||
| Ref 537256 | Synthesis of analogs of forodesine HCl, a human purine nucleoside phosphorylase inhibitor-Part I. Bioorg Med Chem Lett. 2009 May 15;19(10):2624-6. Epub 2009 Apr 9. | ||||
| Ref 537497 | Forodesine has high anti-tumor activity in chronic lymphocytic leukemia and activates p53-independent mitochondrial apoptosis via induction of p73 and BIM. Blood. 2009 Jun 18. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

