Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04EON
|
||||
| Former ID |
DIB010565
|
||||
| Drug Name |
BAL-101553
|
||||
| Indication | Cancer [ICD9: 140-229; ICD10:C00-C96] | Phase 1/2 | [525257] | ||
| Company |
Basilea Pharmaceutica International Ltd
|
||||
| Structure |
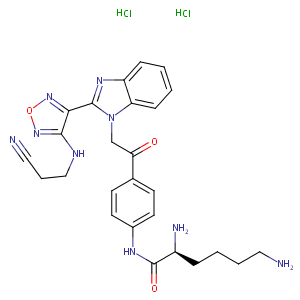
|
Download2D MOL |
|||
| Canonical SMILES |
Cl.Cl.c1(c(non1)NCCC#N)c1n(c2ccccc2n1)CC(=O)c1ccc(cc1)N<br />C(=O)[C@@H](N)CCCCN
|
||||
| Target and Pathway | |||||
| Target(s) | Tubulin beta | Target Info | Inhibitor | [533035] | |
| PANTHER Pathway | Cytoskeletal regulation by Rho GTPase | ||||
| Huntington disease | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

