Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04XCO
|
||||
| Former ID |
DIB008903
|
||||
| Drug Name |
AZD-8848
|
||||
| Synonyms |
AZ-12441970; DSP-3025; SM-324405; TLR7 agonist (allergy), Dainippon/AstraZeneca
|
||||
| Indication | Allergic rhinitis [ICD9: 472.0, 477, 995.3; ICD10:J00, J30, J31.0, T78.4] | Phase 2 | [523140] | ||
| Company |
Dainippon Sumitomo Pharma Co Ltd
|
||||
| Structure |
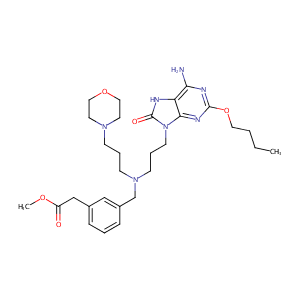
|
Download2D MOL |
|||
| Canonical SMILES |
N(Cc1cc(ccc1)CC(=O)OC)(CCCN1CCOCC1)CCCn1c2nc(nc(c2[nH]c<br />1=O)N)OCCCC
|
||||
| Target and Pathway | |||||
| Target(s) | Toll-like receptor 7 | Target Info | Agonist | [531949] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | Toll receptor signaling pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

