Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00PJJ
|
||||
| Former ID |
DIB011252
|
||||
| Drug Name |
FK-614
|
||||
| Synonyms |
ATx08-001; ATx08-001); PPAR gamma agonist (oral, neuropathic pain), Aestus
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 2 | [527702] | ||
| Company |
Fujisawa Pharmaceutical Co Ltd; aestus therapeutics
|
||||
| Structure |
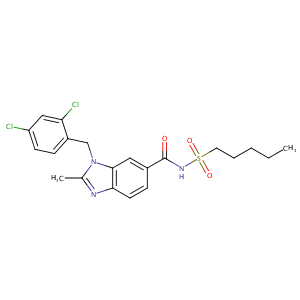
|
Download2D MOL |
|||
| Formula |
C21H23Cl2N3O3S
|
||||
| Canonical SMILES |
n1(c(nc2c1cc(C(=O)NS(=O)(=O)CCCCC)cc2)C)Cc1c(cc(cc1)Cl)<br />Cl
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Peroxisome proliferator activated receptor gamma | Target Info | Agonist | [527702] | |
| PANTHER Pathway | CCKR signaling map ST | ||||
| WikiPathways | Wnt Signaling Pathway Netpath | ||||
| Nuclear Receptors in Lipid Metabolism and Toxicity | |||||
| Differentiation of white and brown adipocyte | |||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | |||||
| Transcriptional Regulation of White Adipocyte Differentiation | |||||
| Adipogenesis | |||||
| SREBP signalling | |||||
| Nuclear Receptors | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

