Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0M8VL
|
||||
| Former ID |
DIB013300
|
||||
| Drug Name |
GW-870086-X
|
||||
| Synonyms |
Glucocorticoid agonist (asthma), GlaxoSmithKline; GW-870086-X (inhaled, asthma), GlaxoSmithKline
|
||||
| Company |
GlaxoSmithKline plc
|
||||
| Structure |
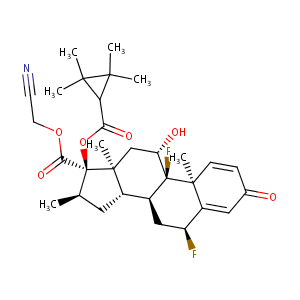
|
Download2D MOL |
|||
| Canonical SMILES |
CC1(C)C(C)(C)C1C(=O)O[C@@]1(C(=O)OCC#N)[C@]2(C[C@@H]([C<br />@@]3([C@]4(C=CC(=O)C=C4[C@H](C[C@H]3[C@@H]2C[C@H]1C)F)C<br />)F)O)C
|
||||
| Target and Pathway | |||||
| Target(s) | Glucocorticoid receptor | Target Info | Agonist | [532254] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TCR Signaling Pathway | |||||
| References | |||||
| Ref 522149 | ClinicalTrials.gov (NCT00549497) A Randomized Study Evaluating Steroid Hormone Levels, Safety And Tolerability Of GW870086X In Healthy Volunteers. U.S. National Institutes of Health. | ||||
| Ref 523364 | ClinicalTrials.gov (NCT01299610) A Study to Test the Effect of 2 Different Doses of Topical GW870086X on Atopic Dermatitis Also Including a Postive Control and a Placebo. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

