Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00MXN
|
||||
| Former ID |
DIB012116
|
||||
| Drug Name |
MK-3207
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Migraine [ICD9: 346; ICD10:G43] | Phase 2 | [522371] | ||
| Company |
Merck & Co Inc
|
||||
| Structure |
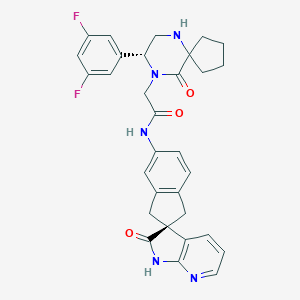
|
Download2D MOL |
|||
| Formula |
C31H30ClF2N5O3
|
||||
| Canonical SMILES |
C12(CCCC1)NC[C@H](N(C2=O)CC(=O)Nc1cc2c(cc1)C[C@@]1(C(=O<br />)Nc3c1cccn3)C2)c1cc(cc(c1)F)F
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Calcitoningene-related peptide type 1 receptor | Target Info | Antagonist | [530631] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

