Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0EJ6O
|
||||
| Former ID |
DAP001359
|
||||
| Drug Name |
Ciprofibrate
|
||||
| Synonyms |
Ciprofibrato; Ciprofibratum; Ciprol; Hiperlipen; Hyperlipen; Lipanor; Modalim; Oroxadin; Sanofi Synthelabo brand of ciprofibrate; Sanofi Winthrop brand of ciprofibrate; C 0330; WIN 35833; Ciprofibrato [INN-Spanish]; Ciprofibratum [INN-Latin]; Win 35,833; Win-35833; Ciprofibrate (USAN/INN); Ciprofibrate [USAN:BAN:INN]; 2-(4-(2,2-Dichlorocyclopropyl)phenoxy)2-methylpropanoic acid; 2-(p-(2,2-Dichlorocyclopropyl)phenoxy)-2-methylpropionic acid; 2-[4-(2,2-Dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid; 2-[p-(2,2-Dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid; 2-{4-[2,2-dichlorocyclopropyl]phenoxy}-2-methylpropanoic acid; 2-{[4-(2,2-dichlorocyclopropyl)phenyl]oxy}-2-methylpropanoic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Structure |
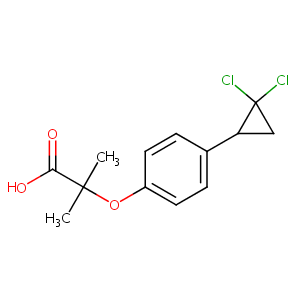
|
Download2D MOL |
|||
| Formula |
C13H14Cl2O3
|
||||
| InChI |
InChI=1S/C13H14Cl2O3/c1-12(2,11(16)17)18-9-5-3-8(4-6-9)10-7-13(10,14)15/h3-6,10H,7H2,1-2H3,(H,16,17)
|
||||
| InChIKey |
KPSRODZRAIWAKH-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 52214-84-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
4816527, 7978950, 8151784, 10852034, 11467104, 11468224, 11486732, 12013723, 14775650, 17397655, 17404731, 24278288, 26757866, 28325632, 29221918, 47291293, 47291294, 47365389, 47736670, 47959950, 48413469, 48415783, 49699279, 49857263, 50105908, 50105909, 50889815, 53777274, 53789241, 56311292, 56312796, 56312797, 56352999, 56463556, 57321443, 85175392, 85230953, 85788082, 90340632, 91011906, 92125998, 92303711, 92713293, 103689672, 103914561, 104301538, 117868533, 121360841, 121363669, 124749506
|
||||
| ChEBI ID |
ChEBI:50867
|
||||
| SuperDrug ATC ID |
C10AB08
|
||||
| SuperDrug CAS ID |
cas=052214843
|
||||
| Target and Pathway | |||||
| Target(s) | Peroxisome proliferator activated receptor alpha | Target Info | Agonist | [534997] | |
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | ||||
| Reactome | RORA activates gene expression | ||||
| BMAL1:CLOCK,NPAS2 activates circadian gene expression | |||||
| PPARA activates gene expression | |||||
| YAP1- and WWTR1 (TAZ)-stimulated gene expression | |||||
| Transcriptional activation of mitochondrial biogenesis | |||||
| Activation of gene expression by SREBF (SREBP) | |||||
| Transcriptional regulation of white adipocyte differentiation | |||||
| Nuclear Receptor transcription pathway | |||||
| Regulation of lipid metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | |||||
| Circadian Clock | |||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | ||||
| Nuclear Receptors Meta-Pathway | |||||
| Estrogen Receptor Pathway | |||||
| PPAR Alpha Pathway | |||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | |||||
| Transcriptional Regulation of White Adipocyte Differentiation | |||||
| YAP1- and WWTR1 (TAZ)-stimulated gene expression | |||||
| Activation of Gene Expression by SREBP (SREBF) | |||||
| Adipogenesis | |||||
| SREBF and miR33 in cholesterol and lipid homeostasis | |||||
| Circadian Clock | |||||
| Nuclear Receptors | |||||
| References | |||||
| Ref 540377 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3438). | ||||
| Ref 550550 | Antidiabetic action of bezafibrate in a large observational database. Diabetes Care. 2009 Apr;32(4):547-51. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

