Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0K6GZ
|
||||
| Former ID |
DAP000161
|
||||
| Drug Name |
Sparfloxacin
|
||||
| Synonyms |
Esparfloxacino; SPFX; Spara; Sparfloxacine; Sparfloxacinum; Zagam; AT 4140; CP 103826; PD 131501; PD131501; AT-4140; CP-103826; DRG-0143; Esparfloxacino [INN-Spanish]; Liposome-encapsulated sparfloxacin; PD 1315-1; PD-131501; RP-64206; Respipac (TN); Sparfloxacin & RU 40555; Sparfloxacine [INN-French]; Sparfloxacinum [INN-Latin]; Zagam (TN); Sparfloxacin, cis-isomer; Sparfloxacin (JAN/USAN/INN); Sparfloxacin [USAN:BAN:INN:JAN]; Cis-5-Amino-1-cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; (cis)-5-amino-1-cyclopropyl-7-(3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; 5-Amino-1-cyclohexyl-7-(cis-3,5-dimethylpiperazino)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; 5-Amino-1-cyclopropyl-7-(cis-3,5-dimethyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid & RU 40555; 5-Amino-1-cyclopropyl-7-(cis-3,5-dimethyl-1-piperazinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid; 5-amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 5-amino-1-cyclopropyl-7-[(3R,5S)-3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxoquinoline-3-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [538553] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Square pharma
|
||||
| Structure |
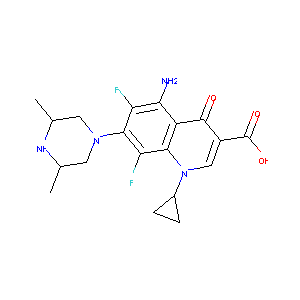
|
Download2D MOL |
|||
| Formula |
C19H22F2N4O3
|
||||
| Canonical SMILES |
CC1CN(CC(N1)C)C2=C(C3=C(C(=C2F)N)C(=O)C(=CN3C4CC4)C(=O)<br />O)F
|
||||
| InChI |
1S/C19H22F2N4O3/c1-8-5-24(6-9(2)23-8)17-13(20)15(22)12-16(14(17)21)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6,22H2,1-2H3,(H,27,28)/t8-,9+
|
||||
| InChIKey |
DZZWHBIBMUVIIW-DTORHVGOSA-N
|
||||
| CAS Number |
CAS 110871-86-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9864, 602967, 603315, 627689, 7847656, 7980640, 8147220, 8186880, 11528734, 12014309, 14830034, 16618671, 24860661, 24880518, 25623210, 26719819, 29218052, 43117868, 46386584, 46506453, 48416549, 56313707, 57314019, 57648946, 57651613, 78600880, 85279509, 92309275, 92710927, 103223807, 104225171, 104320846, 117393504, 124766044, 124893258, 125325629, 126631379, 126682799, 127811456, 131294555, 131905130, 134223183, 134337481, 135018692, 137003456, 142971059, 144205133, 160845924, 160964541, 163414061
|
||||
| ChEBI ID |
ChEBI:9212
|
||||
| SuperDrug ATC ID |
J01MA09
|
||||
| SuperDrug CAS ID |
cas=110871868
|
||||
| Target and Pathway | |||||
| Target(s) | Bacterial DNA gyrase | Target Info | Modulator | [556264] | |
| Topoisomerase IV | Target Info | Modulator | [556264] | ||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

