Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00HCQ
|
||||
| Former ID |
DAP000761
|
||||
| Drug Name |
Capecitabine
|
||||
| Synonyms |
Capecitabin; Capecitabina; Capecitabinum; Capecitibine; Capiibine; Caxeta; Xabine; Xeloda; Capecitabine [USAN]; R340;R-340; RG-340; Ro 09-1978; Xeloda (TN); Ro 09-1978/000; Ro-09-1978; Xeloda, Captabin, Capecitabine; Capecitabine (JAN/USAN/INN); Ro-09-1978/000; N(4)-Pentyloxycarbonyl-5'-deoxy-5-fluorocytidine; Pentyl 1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinecarbamate; Pentyl [1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]carbamate; Carbamic acid, (1-(5-deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-, pentyl ester; Pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate; (1-(5-Deoxy-beta-D-ribofuranosyl)-5-fluoro-1,2-dihydro-2-oxo-4-pyrimidinyl)-carbamic acid pentyl ester; 5'-Deoxy-5-fluoro-N-((pentyloxy)carbonyl)cytidine; 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine; Capecitabine (Fluoropyrimidine)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Roche
|
||||
| Structure |
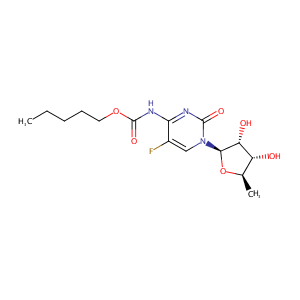
|
Download2D MOL |
|||
| Formula |
C15H22FN3O6
|
||||
| InChI |
InChI=1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1
|
||||
| InChIKey |
GAGWJHPBXLXJQN-UORFTKCHSA-N
|
||||
| CAS Number |
CAS 154361-50-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
583040, 7848286, 7978485, 8187136, 12014895, 14828191, 14925873, 29214852, 43118267, 46508686, 49960125, 53790473, 57314195, 71821403, 74459733, 99436918, 103724218, 104322032, 117506114, 117673331, 118048469, 119526524, 124757042, 125163846, 126653765, 126671131, 127925204, 134338046, 134358377, 135017419, 135693779, 135723537, 136368008, 136375551, 136949108, 137005622, 140014282, 143493385, 144115785, 144205751, 152059549, 152235778, 152258963, 160647810, 160964435, 162011675, 163304897, 164175281, 164194987, 165245546
|
||||
| ChEBI ID |
ChEBI:31348
|
||||
| SuperDrug ATC ID |
L01BC06
|
||||
| SuperDrug CAS ID |
cas=154361509
|
||||
| Target and Pathway | |||||
| Target(s) | Thymidylate synthase | Target Info | Inhibitor | [536046] | |
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| Ref 536361 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| Ref 541880 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6799). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

