| Drug General Information |
| Drug ID |
D0ZS3M
|
| Former ID |
DNC012018
|
| Drug Name |
Indol-1-yl-prop-2-ynyl-pyridin-4-yl-amine
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
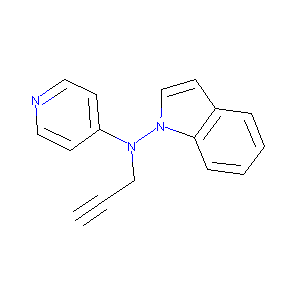
|
Download
2D MOL
3D MOL
|
| Formula |
C16H13N3
|
| Canonical SMILES |
C#CCN(C1=CC=NC=C1)N2C=CC3=CC=CC=C32
|
| InChI |
1S/C16H13N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h1,3-11,13H,12H2
|
| InChIKey |
KUBLGYWISNGZNK-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
Alpha-2A adrenergic receptor |
Target Info |
Inhibitor |
[1]
|
|---|
| Alpha-2B adrenergic receptor |
Target Info |
Inhibitor |
[1]
|
| Alpha-2C adrenergic receptor |
Target Info |
Inhibitor |
[1]
|
|
KEGG Pathway
|
cGMP-PKG signaling pathway
|
|
Neuroactive ligand-receptor interactionhsa04022:cGMP-PKG signaling pathway
|
|
Neuroactive ligand-receptor interaction
|
|
PANTHER Pathway
|
Alpha adrenergic receptor signaling pathwayP00002:Alpha adrenergic receptor signaling pathway
|
|
Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathwayP00002:Alpha adrenergic receptor signaling pathway
|
|
Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway
|
|
Reactome
|
Adrenoceptors
|
|
Adrenaline signalling through Alpha-2 adrenergic receptor
|
|
Adrenaline,noradrenaline inhibits insulin secretion
|
|
G alpha (i) signalling events
|
|
G alpha (z) signalling events
|
|
Surfactant metabolismR-HSA-390696:Adrenoceptors
|
|
G alpha (z) signalling eventsR-HSA-390696:Adrenoceptors
|
|
Surfactant metabolism
|
|
WikiPathways
|
Monoamine GPCRs
|
|
GPCRs, Class A Rhodopsin-like
|
|
Platelet Aggregation (Plug Formation)
|
|
Integration of energy metabolism
|
|
GPCR ligand binding
|
|
GPCR downstream signalingWP58:Monoamine GPCRs
|
|
GPCR downstream signaling
|
| References |
| REF 1 | J Med Chem. 1996 Jan 19;39(2):570-81.Synthesis and structure-activity relationships of N-propyl-N-(4-pyridinyl)-1H-indol-1-amine (besipirdine) and related analogs as potential therapeutic agents for Alzheimer's disease. |
|---|