Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y4GO
|
||||
| Former ID |
DAP000049
|
||||
| Drug Name |
Pemetrexed
|
||||
| Synonyms |
Alimta; LYA; LY 231514; LY231514; Alimta (TN); LY 231,514; LY-2315; LY-231514; Pemetrexed (INN); Pemetrexed [INN:BAN]; LY-231,514; N-(4-(2-(2-Amino-3,4-dihydro-4-oxo-7H-pyrrolo(2,3-d)pyrimdin-5-yl)ethyl)benzoyl)glutamic acid; N-{4-[2-(2-amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-d-glutamic acid; (2R)-2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid; (2S)-2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid; 2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid; 2-{4-[2-(2-AMINO-4-OXO-4,7-DIHYDRO-3H-PYRROLO[2,3-D]PYRIMIDIN-5-YL)-ETHYL]-BENZOYLAMINO}-PENTANEDIOIC ACID
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Eli Lilly
|
||||
| Structure |
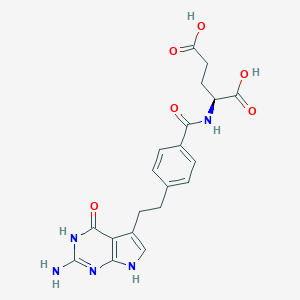
|
Download2D MOL |
|||
| Formula |
C20H21N5O6
|
||||
| InChI |
InChI=1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t13-/m1/s1
|
||||
| InChIKey |
WBXPDJSOTKVWSJ-CYBMUJFWSA-N
|
||||
| CAS Number |
CAS 150399-23-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
43118181, 46505640, 50064379, 50524509, 53788923, 57314144, 90342403, 104321790, 117542033, 126665655, 134337377, 134338975, 134339784, 134457174, 135945851, 137002681, 152035380, 160963987, 175437774, 177748645, 178103443, 189306395, 223392020, 223658518, 223705327, 224164966, 226399254, 250180319, 252216021
|
||||
| SuperDrug ATC ID |
L01BA04
|
||||
| SuperDrug CAS ID |
cas=137281233
|
||||
| Target and Pathway | |||||
| Target(s) | Thymidylate synthase | Target Info | Inhibitor | [537219] | |
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| Ref 527466 | 2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4. | ||||
| Ref 531376 | Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer. 2011 Oct;74(1):132-8. | ||||
| Ref 537580 | Pemetrexed in the treatment of advanced non-squamous lung cancer. Lung Cancer. 2009 Jul 3. | ||||
| Ref 541917 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6837). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

