Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y1MW
|
||||
| Former ID |
DNC000558
|
||||
| Drug Name |
Difluoromethylornithine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Trypanosoma brucei gambiense infection [ICD9: 86.5; ICD10:B56] | Phase 2 | [523970] | ||
| Structure |
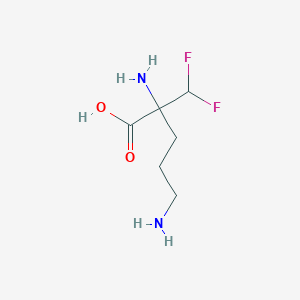
|
Download2D MOL |
|||
| Formula |
C6H13F2N2O2+
|
||||
| Canonical SMILES |
C(CC(C(F)F)(C(=O)[O-])[NH3+])C[NH3+]
|
||||
| InChI |
1S/C6H12F2N2O2/c7-4(8)6(10,5(11)12)2-1-3-9/h4H,1-3,9-10H2,(H,11,12)/p+1
|
||||
| InChIKey |
VLCYCQAOQCDTCN-UHFFFAOYSA-O
|
||||
| CAS Number |
CAS 67037-37-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| ChEBI ID |
ChEBI:41948
|
||||
| SuperDrug ATC ID |
P01CX03
|
||||
| SuperDrug CAS ID |
cas=070052129
|
||||
| Target and Pathway | |||||
| Target(s) | Ornithine decarboxylase | Target Info | Inhibitor | [537878] | |
| Arginase II, mitochondrial | Target Info | Inhibitor | [535841] | ||
| BioCyc Pathway | Putrescine biosynthesis I | ||||
| PANTHER Pathway | Ornithine degradation | ||||
| CCKR signaling map ST | |||||
| Pathway Interaction Database | Validated targets of C-MYC transcriptional activation | ||||
| PathWhiz Pathway | Spermidine and Spermine Biosynthesis | ||||
| References | |||||
| Ref 535841 | Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003 Oct 21;108(16):2000-6. Epub 2003 Sep 29. | ||||
| Ref 537878 | An inhibitor of polyamine biosynthesis impairs human polymorphonuclear leukocyte priming by tumor necrosis factor alpha. J Leukoc Biol. 1995 Feb;57(2):282-6. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

