Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V9HH
|
||||
| Former ID |
DIB013145
|
||||
| Drug Name |
Taprizosin
|
||||
| Synonyms |
UK-338003
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Prostate hyperplasia [ICD10:N40] | Discontinued in Phase 2 | [1] | ||
| Company |
Pfizer Inc
|
||||
| Structure |
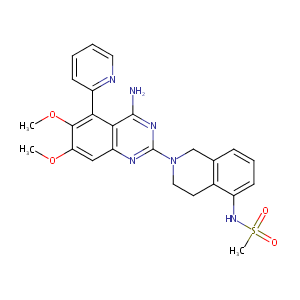
|
Download2D MOL |
|||
| Formula |
C25H26N6O4S
|
||||
| Canonical SMILES |
n1c(nc2c(c(c(c(c2)OC)OC)c2ncccc2)c1N)N1Cc2c(c(NS(=O)(=O<br />)C)ccc2)CC1
|
||||
| CAS Number |
CAS 210538-44-6
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Alpha-1A adrenergic receptor | Target Info | Antagonist | [2] | |
| KEGG Pathway | Calcium signaling pathway | ||||
| cGMP-PKG signaling pathway | |||||
| Neuroactive ligand-receptor interaction | |||||
| AMPK signaling pathway | |||||
| Adrenergic signaling in cardiomyocytes | |||||
| Vascular smooth muscle contraction | |||||
| Salivary secretion | |||||
| PANTHER Pathway | Alpha adrenergic receptor signaling pathway | ||||
| Reactome | Adrenoceptors | ||||
| G alpha (q) signalling events | |||||
| G alpha (12/13) signalling events | |||||
| WikiPathways | Monoamine GPCRs | ||||
| Calcium Regulation in the Cardiac Cell | |||||
| GPCRs, Class A Rhodopsin-like | |||||
| Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| Endothelin Pathways | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| AMPK Signaling | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017098) | ||||
| REF 2 | Impact of physicochemical and structural properties on the pharmacokinetics of a series of alpha1L-adrenoceptor antagonists. Drug Metab Dispos. 2007 Aug;35(8):1435-45. Epub 2007 May 14. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

