Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0U1US
|
||||
| Former ID |
DIB002686
|
||||
| Drug Name |
18F-FEDAA-1106
|
||||
| Synonyms |
ZK-6032924; FEDAA-1106-[18F]; Fluorine-18-FEDAA-1106; 18F-labeled PET imaging agent (Alzheimer's disease), Bayer; 18F-labeled PET imaging agent (multiple sclerosis), Bayer
|
||||
| Indication | Alzheimer disease [ICD9: 331; ICD10:G30] | Phase 1 | [549054] | ||
| Company |
Taisho Pharmaceutical Co Ltd
|
||||
| Structure |
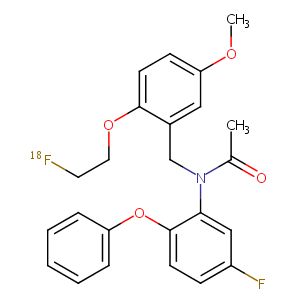
|
Download2D MOL |
|||
| Canonical SMILES |
c1(c(Oc2ccccc2)ccc(c1)F)N(Cc1c(ccc(c1)OC)OCC[18F])C(=O)<br />C
|
||||
| Target and Pathway | |||||
| Target(s) | Peripheral-type benzodiazepine receptor | Target Info | Modulator | [526694] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| HTLV-I infection | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

