Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T5PO
|
||||
| Former ID |
DNC003593
|
||||
| Drug Name |
ROLIPRAM
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Phase 2 | [1], [2] | ||
| Structure |
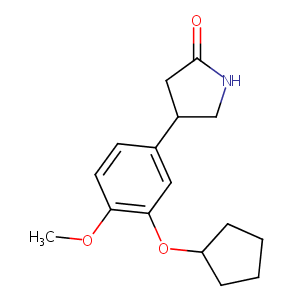
|
Download2D MOL |
|||
| Formula |
C16H21NO3
|
||||
| InChI |
InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18)
|
||||
| InChIKey |
HJORMJIFDVBMOB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 61413-54-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
611179, 841134, 855869, 3153149, 5146349, 7848845, 7980298, 8153132, 11121421, 11121901, 11362490, 11365052, 11367614, 11370251, 11370252, 11373215, 11375776, 11466952, 11468072, 11486743, 12013590, 15222043, 17405628, 22391521, 24278196, 26681488, 26719663, 26719664, 26752319, 26752320, 26752321, 26759453, 29224160, 46386779, 46386848, 46487946, 46514702, 47217030, 47365441, 47440514, 47811011, 48035380, 48185221, 48334758, 49635712, 49699213, 50039658, 50105263, 50105264, 50105265
|
||||
| Target and Pathway | |||||
| Target(s) | CAMP-specific 3',5'-cyclic phosphodiesterase 4B | Target Info | Inhibitor | [3] | |
| Type IV phosphodiesterase | Target Info | Inhibitor | [4] | ||
| CGMP-specific 3',5'-cyclic phosphodiesterase | Target Info | Inhibitor | [5] | ||
| CAMP-specific 3',5'-cyclic phosphodiesterase 4A | Target Info | Inhibitor | [6] | ||
| KEGG Pathway | Purine metabolism | ||||
| cAMP signaling pathway | |||||
| Morphine addictionhsa00230:Purine metabolism | |||||
| cGMP-PKG signaling pathwayhsa00230:Purine metabolism | |||||
| Morphine addiction | |||||
| NetPath Pathway | IL5 Signaling Pathway | ||||
| IL2 Signaling PathwayNetPath_7:TGF_beta_Receptor Signaling Pathway | |||||
| PathWhiz Pathway | Purine Metabolism | ||||
| Reactome | DARPP-32 events | ||||
| G alpha (s) signalling eventsR-HSA-180024:DARPP-32 events | |||||
| G alpha (s) signalling eventsR-HSA-418457:cGMP effectsR-HSA-180024:DARPP-32 events | |||||
| G alpha (s) signalling events | |||||
| WikiPathways | G Protein Signaling Pathways | ||||
| Myometrial Relaxation and Contraction Pathways | |||||
| Nuclear Receptors Meta-Pathway | |||||
| Opioid SignallingWP35:G Protein Signaling Pathways | |||||
| TSH signaling pathwayWP35:G Protein Signaling Pathways | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00011375) Rolipram to Treat Multiple Sclerosis. U.S. National Institutes of Health. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5260). | ||||
| REF 3 | J Med Chem. 1997 May 9;40(10):1417-21.Novel heterocyclic-fused pyridazinones as potent and selective phosphodiesterase IV inhibitors. | ||||
| REF 4 | Bioorg Med Chem. 2010 Mar 15;18(6):2204-18. Epub 2010 Feb 8.In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. | ||||
| REF 5 | J Med Chem. 1994 Jun 24;37(13):2106-11.Cyclic GMP phosphodiesterase inhibitors. 2. Requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. | ||||
| REF 6 | Discovery of micromolar PDE IV inhibitors that exhibit much reduced affinity for the [3H]rolipram binding site: 3-norbornyloxy-4-methoxyphenylmethylene oxindoles, Bioorg. Med. Chem. Lett. 5(17):1965-1968 (1995). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

