Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T1WN
|
||||
| Former ID |
DIB008666
|
||||
| Drug Name |
Zolpidem
|
||||
| Synonyms |
ZolpiMist; Zolpidem tartrate; Zolpidem (low-dose oral spray, middle-of-the-night awakenings); Zolpidem (low-dose oral spray, middle-of-the-night awakenings), NovaDel
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Insomnia [ICD9: 307.41, 307.42, 327.0, 780.51, 780.52; ICD10:F51.0, G47.0] | Approved | [1], [2] | ||
| Sleep maintenance insomnia [ICD9: 307.41, 307.42, 327.0, 780.51, 780.52; ICD10:F51.0, G47.0] | Phase 1 | [3] | |||
| Company |
NovaDel Pharma Inc
|
||||
| Structure |
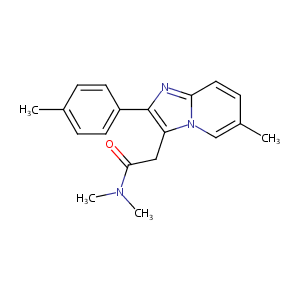
|
Download2D MOL |
|||
| Formula |
C19H21N3O
|
||||
| InChI |
InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3
|
||||
| InChIKey |
ZAFYATHCZYHLPB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 99294-93-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9428, 5375169, 7980918, 8153513, 10536652, 14874110, 24902172, 26612931, 26749064, 26749065, 29224769, 46507949, 48185081, 49981229, 50071297, 50107516, 50107517, 50575529, 53789442, 56464703, 57322919, 58107084, 81040955, 85789644, 87322637, 92124686, 93166943, 94568979, 96025373, 103238173, 104310097, 117870887, 125240940, 125358199, 126525320, 126645781, 126684070, 129873686, 131842757, 134337437, 135019994, 135584272, 135697995, 136974156, 137003607, 142409082, 144205024, 160963771, 163109116, 164196673
|
||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor subunit alpha-1 | Target Info | Modulator | ||
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | |||||
| GABAergic synapse | |||||
| Morphine addiction | |||||
| Nicotine addiction | |||||
| Reactome | Ligand-gated ion channel transport | ||||
| GABA A receptor activation | |||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transport | |||||
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4348). | ||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 3 | Improved insomnia symptoms and sleep-related next-day functioning in patients with comorbid major depressive disorder and insomnia following concomitant zolpidem extended-release 12.5 mg and escitalopram treatment: a randomized controlled trial. J Clin Psychiatry. 2011 Jul;72(7):914-28. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

