Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0R2SK
|
||||
| Former ID |
DIB007558
|
||||
| Drug Name |
Erteberel
|
||||
| Synonyms |
LY-500307; SERBA-1; ER beta agonist (BPH), Lilly; Lead estrogen receptor beta agonists (benign prostatic hyperplasia),Eli Lilly
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Prostate hyperplasia [ICD10:N40] | Phase 2 | [1] | ||
| Company |
Eli Lilly & Co
|
||||
| Structure |
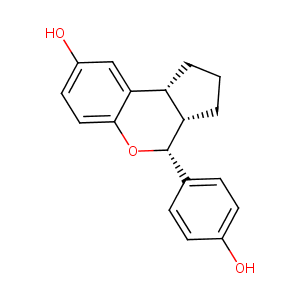
|
Download2D MOL |
|||
| Formula |
C18H18O3
|
||||
| Canonical SMILES |
[C@H]12[C@H](c3c(O[C@H]1c1ccc(cc1)O)ccc(c3)O)CCC2
|
||||
| CAS Number |
CAS 533884-09-2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Estrogen receptor beta | Target Info | Agonist | [2] | |
| KEGG Pathway | Estrogen signaling pathway | ||||
| Prolactin signaling pathway | |||||
| Pathway Interaction Database | Plasma membrane estrogen receptor signaling | ||||
| Validated nuclear estrogen receptor beta network | |||||
| Validated nuclear estrogen receptor alpha network | |||||
| Reactome | Nuclear Receptor transcription pathway | ||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Ovarian Infertility Genes | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| Nuclear Receptors | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01874756) The Efficacy and Safety of a Selective Estrogen Receptor Beta Agonist (LY500307) for Negative Symptoms and Cognitive Impairment Associated With Schizophrenia. U.S. National Institutes of Health. | ||||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031986) | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

