Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Q2PE
|
||||
| Former ID |
DAP000654
|
||||
| Drug Name |
Norfloxacin
|
||||
| Synonyms |
Baccidal; Barazan; Chibroxin; Fulgram; Lexinor; NFLX; Norflo; Norfloxacine; Norfloxacino; Norfloxacinum; Noroxin; Sebercim; Merck Brand of Norfloxacin; Norfloxacin Merck Brand; AM 0715; AM 715; AM0715; MK 0366; MK 366; MK0366; MK366; AM-0715; AM-715; Chibroxin (TN); Floxin (TN); Insensye (TN); MK-0366; MK-366; Norflohexal (TN); Norfloxacine [INN-French]; Norfloxacino [INN-Spanish]; Norfloxacinum [INN-Latin]; Norfocin (TN); Noroxin (TN); Nufloxib (TN); Roxin (TN); Utin (TN); Utinor (TN); Apo-Norflox (TN); Norfloxacin (JP15/USP/INN); Norfloxacin [USAN:BAN:INN:JAN]; Chibroxin, MK-366, Baccidal, Sebercim, Zoroxin, Norfloxacin; 1,4-Dihydro-1-ethyl-6-fluoro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid; 1-Ethyl-6-fluor-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-chinolincarbonsaeure; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid; 1-Ethyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-piperazinyl]-3-quinoline-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carboxylic acid; 1-ethyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [538281] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Structure |
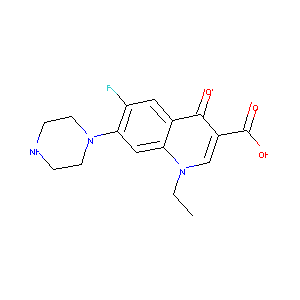
|
Download2D MOL |
|||
| Formula |
C16H18FN3O3
|
||||
| Canonical SMILES |
CCN1C=C(C(=O)C2=CC(=C(C=C21)N3CCNCC3)F)C(=O)O
|
||||
| InChI |
1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23)
|
||||
| InChIKey |
OGJPXUAPXNRGGI-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 70458-96-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
8912, 597742, 855614, 3205552, 4712110, 7847277, 7980171, 8149451, 8152796, 10321598, 11112831, 11335259, 11360498, 11363911, 11366473, 11369035, 11371707, 11374005, 11377197, 11461470, 11466249, 11467369, 11484909, 11485918, 11488833, 11490340, 11492268, 11494831, 12013332, 14715876, 14874649, 26611845, 26680206, 26746929, 26746930, 29223630, 46508634, 47440088, 47440089, 47440090, 47515159, 47736303, 47736304, 47810598, 47959570, 47959571, 48034947, 49698389, 50042536, 50100528
|
||||
| SuperDrug ATC ID |
J01MA06; S01AE02
|
||||
| SuperDrug CAS ID |
cas=070458967
|
||||
| Target and Pathway | |||||
| Target(s) | Bacterial DNA gyrase | Target Info | Modulator | [556264] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

